Ketorolac implant and preparation method thereof
A technology of ketorolac and implants, applied in the field of medicine, can solve problems such as difficulty in realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
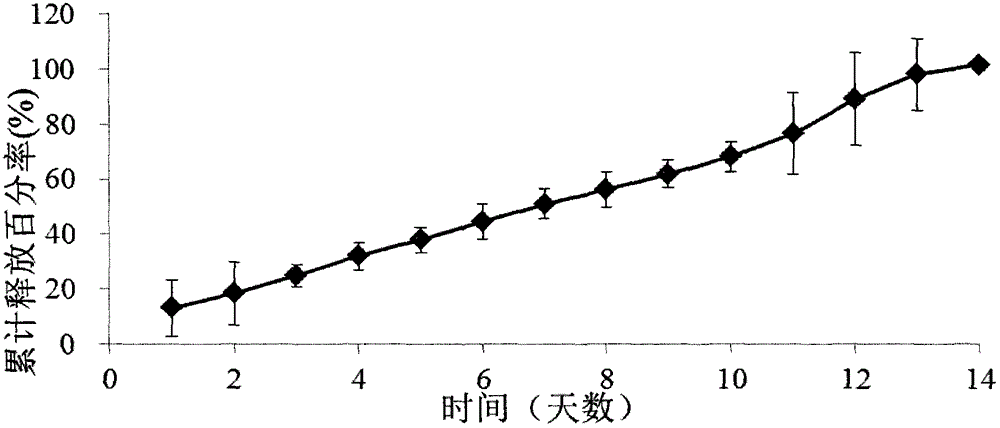
[0067] Example 1: Ketorolac implants (released for 2 days)
[0068] 1) Preparation of ketorolac implant (released for 2 days)
[0069] In terms of weight percentage, 30% of ketorolac tromethamine and 20% of poly D, L-lactic acid (carboxyl terminated, molecular weight 18kDa to 28 kDa) and 40% of poly D, L-lactic acid-CO-ethanol Acid (carboxyl end-capped, ratio of lactic acid to glycolic acid is 50:50, molecular weight range is 7kDa to 17kDa) and 10% polyethylene glycol (PEG4000) are mixed and put into Turbula three-dimensional mixer (T2F type, WAB Machinery Company) and Shake the 4 stainless steel balls twice for 15 minutes each time. Then the mixture was put into a HAAKE mini twin-screw extruder (MiniLab type, Thermo Scientific), the temperature of the extruder was set at 66°C, and the speed was set at 25 revolutions per minute. The extruded rod has a diameter of 0.5mm. Cut the small rod-shaped implant with a blade into small test specimens with a length of 2 mm.
[0070] 2) In v...
Embodiment 2
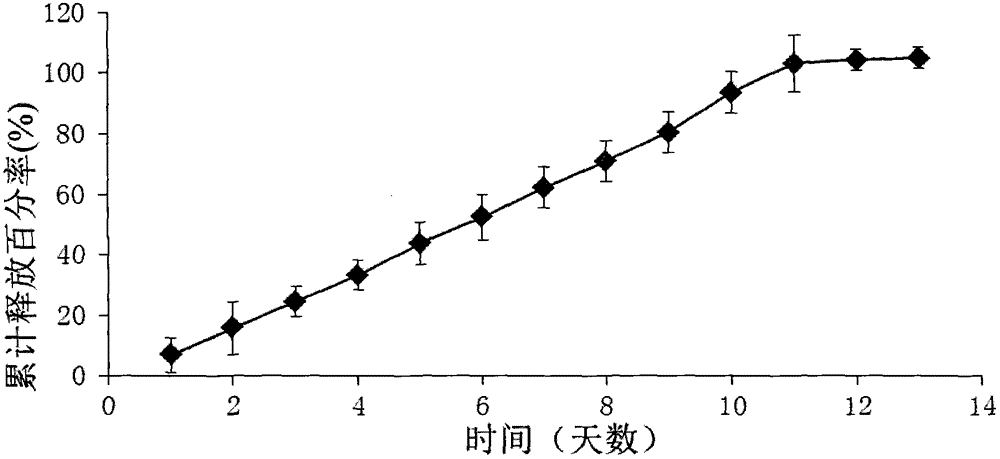
[0074] Example 2: Ketorolac implants (released for 5 days)
[0075] 1) Preparation of ketorolac implant (released for 5 days)
[0076] In terms of weight percentages, 50% ketorolac tromethamine and 30% poly D, L-lactic acid (carboxyl terminated, molecular weight 18kDa to 28 kDa) and 20% poly D, L-lactic acid-CO-ethanol Acid (carboxyl end capped, ratio of lactic acid to glycolic acid is 50:50, molecular weight range is 7kDa to 17kDa), put into Turbula three-dimensional mixer (T2F type, WAB Machinery Company) and 4 stainless steel balls shake twice, 15 times each time minute. The mixture was then put into a HAAKE miniature twin-screw extruder (MiniLab type, Thermo Scientific), the temperature of the extruder was set at 78°C, and the speed was set at 25 revolutions per minute. The extruded rod has a diameter of 0.5mm. Cut the small rod-shaped implant with a blade into small pieces of 2 mm in length.
[0077] 2) In vitro release test:
[0078] Place each test sample (n=4) in an 8 ml g...
Embodiment 3
[0080] Example 3: Ketorolac implant (11 days release)
[0081] 1) Preparation of ketorolac implant (11 days release)
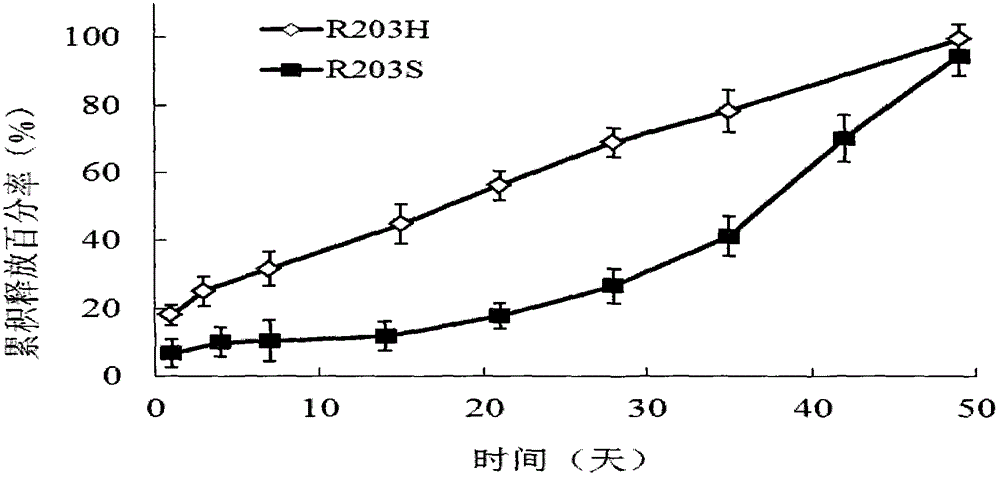
[0082] Five groups of ketorolac implants with a drug release for 11 days were prepared with different contents of ketorolac tromethamine and rate modifiers, as well as different molecular weights and different contents of carboxyl-terminated PLA and carboxyl-terminated PLGA. The details are as follows 2 shows:
[0083] Table 2: Ketorolac implants released for 11 days
[0084]
[0085] The preparation method of implant B is as follows:
[0086] In terms of weight percentages, 40% ketorolac tromethamine and 25% poly D, L-lactic acid (carboxyl terminated, molecular weight 10kDa to 18kDa) and 35% poly D, L-lactic acid-CO-ethanol Acid (carboxyl end capped, ratio of lactic acid to glycolic acid is 50:50, molecular weight range is 7kDa to 17kDa), put into Turbula three-dimensional mixer (T2F type, WAB Machinery Company) and 4 stainless steel balls shake twice, 15 times each ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com