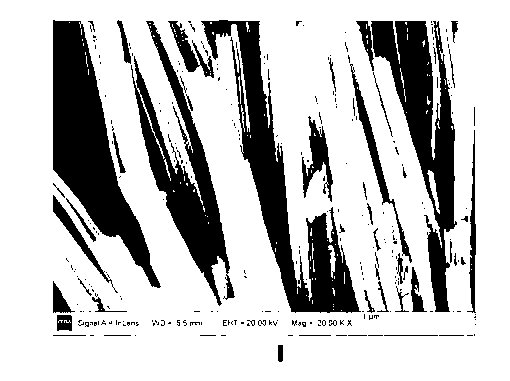
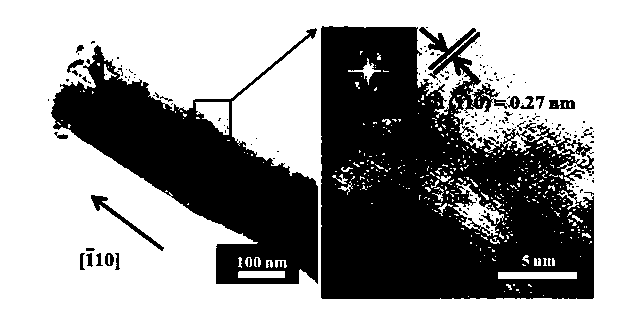
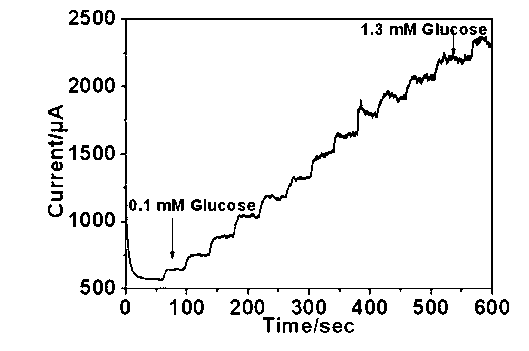
One-dimensional copper oxide nano-array glucose sensor electrode material and preparation method thereof
A glucose sensor, nanoarray technology, applied in copper oxide/copper hydroxide, material analysis by electromagnetic means, nanotechnology, etc., can solve the controllability of difficult material morphology, the operation is not simple enough, and the synthesis yield is not high. Advanced problems, to achieve the effect of good conductivity and electron transport ability, good selectivity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a. Clean the copper sheet with a purity of 99.99% with concentrated hydrochloric acid for 5-10 minutes, then ultrasonically clean it with absolute ethanol or acetone for 10 minutes, then clean it with deionized water, and coat one side (back) of the copper sheet with a layer of insulation Non-reactive materials to avoid contact with the electrolyte during electrolysis below.
[0035] b. take the copper sheet processed in step a as the working electrode; the platinum electrode as the counter electrode; the saturated calomel electrode as the reference electrode, and 50ml of KOH with a concentration of 3.0mol / L as the electrolyte solution, and immerse the copper sheet in the electrolyte solution , with an immersion area of 1.0cm 2 , using the electrochemical workstation to apply a constant current of 3.0mA cm -2 , for anodic polarization reaction;
[0036] c. After 25 minutes of polarization reaction, take out the copper sheet, wash the surface of the electrode with et...
Embodiment 2
[0044] a. Clean the copper sheet with a purity of 99.99% with concentrated hydrochloric acid for 5-10 minutes, then ultrasonically clean it with absolute ethanol or acetone for 10 minutes, then clean it with deionized water, and coat one side (back) of the copper sheet with a layer of insulation Non-reactive materials to avoid contact with the electrolyte during electrolysis below.
[0045] b. take the copper sheet processed in step a as the working electrode; the platinum electrode as the counter electrode; the saturated calomel electrode as the reference electrode, and 50ml of KOH with a concentration of 2.0mol / L as the electrolyte solution, and immerse the copper sheet in the electrolyte solution , with an immersion area of 1.0cm 2 , using the electrochemical workstation to apply a constant current of 2.5mA cm -2 , for the anodic polarization reaction.
[0046] c. After 25 minutes of polarization reaction, take out the copper sheet, wash the surface of the electrode wit...
Embodiment 3
[0051] a. Clean the copper sheet with a purity of 99.99% with concentrated hydrochloric acid for 5-10 minutes, then ultrasonically clean it with absolute ethanol or acetone for 10 minutes, then clean it with deionized water, and coat one side (back) of the copper sheet with a layer of insulation Non-reactive materials to avoid contact with the electrolyte during electrolysis below.
[0052] b. take the copper sheet processed in step a as the working electrode; the platinum electrode as the counter electrode; the saturated calomel electrode as the reference electrode, and 50ml of KOH with a concentration of 1.5mol / L as the electrolyte solution, and immerse the copper sheet in the electrolyte solution , with an immersion area of 1.0cm 2 , using the electrochemical workstation to apply a constant current of 2.0mA cm -2 , for the anodic polarization reaction.
[0053] c. After 20 minutes of polarization reaction, take out the copper sheet, wash the surface of the electrode wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com