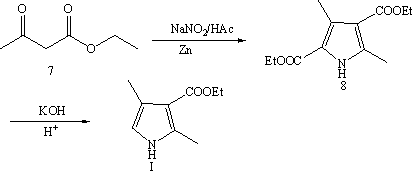
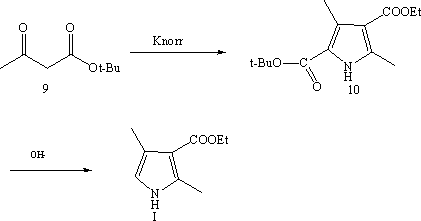
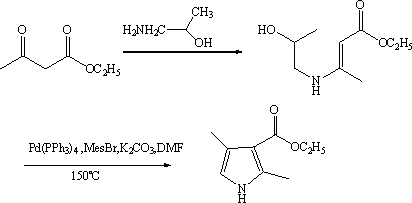
Synthetic method of 2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl ester
A technology of ethyl pyrrolecarboxylate and a synthetic method, applied in the direction of organic chemistry, etc., can solve the problems of difficult control of the hydrolysis process, harsh reaction conditions, cumbersome operation steps, etc., and achieve easy large-scale industrial production, mild reaction conditions, and simple operation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, a kind of synthetic method of ethyl 2,4-dimethyl-3-pyrrolecarboxylate, the method comprises the following steps:
[0029] (1) Propionaldehyde and bromine undergo bromination reaction to obtain 2-bromopropionaldehyde; the reaction is carried out at 0°C~50°C; the solvent used is an aprotic solvent;
[0030] (2) 2-Bromopropionaldehyde, ethyl acetoacetate and ammonia water undergo a ring-closing reaction to obtain ethyl 2,4-dimethyl-3-pyrrolecarboxylate; the reaction is carried out under alkaline conditions at 0°C to 50°C.
Embodiment 2
[0031] Example 2, in the synthesis method described in Example 1: the aprotic solvent described in step (1) is selected from toluene, dichloromethane, N,N-dimethylformamide, N,N-dimethylformamide One or a mixture of amides and dimethyl sulfoxide.
Embodiment 3
[0032] Embodiment 3, in the synthetic method described in embodiment 1 or 2: its specific synthetic steps are as follows:
[0033] (1) Preparation of 2-bromopropionaldehyde: Add propionaldehyde to an appropriate amount of aprotic solvent, cool down to 0-10°C, add bromine dropwise, control the reaction temperature at 0°C-50°C during the dropwise addition, drop bromine After the addition is completed, react at 0°C~50°C for 3~5h, wait until the color of bromine fades, and concentrate to dryness to obtain 2-bromopropionaldehyde; the mass ratio of propionaldehyde to bromine is 1:2.5~3.5;
[0034] (2) Preparation of ethyl 2,4-dimethyl-3-pyrrolecarboxylate: put 2-bromopropionaldehyde and ethyl acetoacetate into the reaction vessel, cool down to 0~10°C, add ammonia water dropwise, and drop the temperature 0°C~50°C. After the dropwise addition, react at 0°C~50°C for 10-14 hours; Remove dichloromethane, freeze and crystallize for 2 days, take out the crystals and stir at 0°C, beat the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com