Multivalent carrier vaccine for crucian carp hemorrhagic disease and its application
A carrier vaccine and hemorrhagic disease technology, applied in the direction of antiviral agents, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve the problems of crucian carp breeding industry impact, prevention and control difficulties, and vaccines are difficult to work, and achieve broad commercial development Prospects, effects of achieving high-efficiency immunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of crucian carp haemorrhagic disease polyvalent carrier vaccine
[0027] 1. Culture medium preparation
[0028] (1) DSM liquid medium: bacterial nutrient broth (Difco) 8g, 10% (w / v) KCl 10ml, 1.2% (w / v) MgSO 4 ·7H 2 O10ml, 1MNaOH1.5ml, adjust pH to 7.6, ddH 2 O was adjusted to 1000ml. After autoclaving, cool to 50°C, add sterilized 1MCa (NO 3 ) 2 , 0.01MMnCl 2 , 1mM FeSO 4 1ml each.
[0029] (2) PBS buffer: Na 2 HPO 4 2H 2 O2.74g / L, NaH 2 PO 4 ·H 2 0.63g / L, the composition was dissolved in 1000ml distilled water, and sterilized at 121°C for 20 minutes.
[0030] (3) LB medium: tryptone (Tryptone) 10g / L, yeast extract (Yeasextract) 5g / L, sodium chloride (NaCl) 10g / L, adjust the pH to 7.0.
[0031] 2. Recovery of the plate medium: take the seeds of the recombinant Bacillus subtilis strain with the preservation number CCTCCNo: M2013029 stored at -80°C and inoculate them on the LB solid plate by streaking and culturing overnight at 37...
Embodiment 2
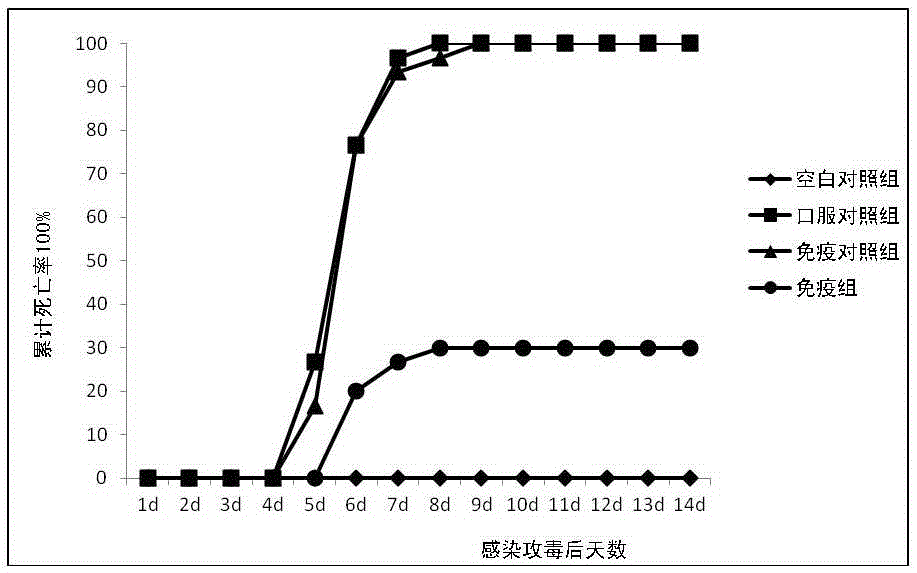
[0036] Example 2: Evaluation of the immune effect of carp herpesvirus II (CyHV-2) on crucian carp immunized with multivalent carrier vaccine orally.
[0037] Breeding of experimental fish: The experimental fish (crucian carp) purchased from Jiangsu was temporarily raised in a 2000L aquarium after being tested for the absence of CyHV-2, equipped with a flow circulation fresh water treatment and purification system. After raising for 1 week, remove unhealthy individuals. Select healthy crucian carp with a weight of 350±50g and randomly group them into 200L experimental aquariums, 30 in each aquarium, change the water twice a day in the experimental aquarium, and change the water volume to 1 / 3, maintain the water temperature at 20±2°C, and continue to raise temporarily 2 weeks.
[0038] Preparation of CyHV-2 virus suspension: dissect diseased fish, select gills, kidney and spleen, cut them into pieces and grind them under liquid nitrogen, add TN buffer (50mmol / LTris-HCl , pH7.6...
Embodiment 3
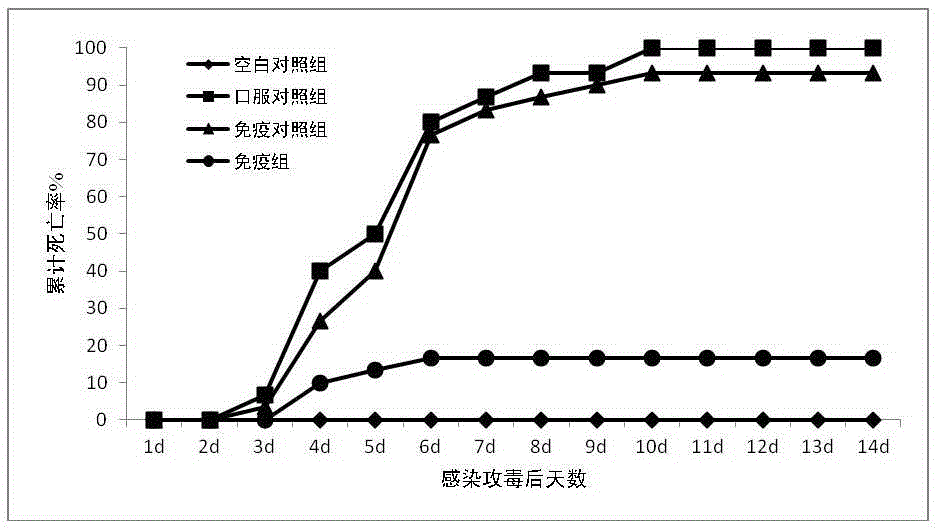
[0044] Example 3: Evaluation of the immune effect of crucian carp immunized orally with multivalent carrier vaccine against Aeromonas hydrophila.
[0045] Breeding of experimental fish: The experimental fish (crucian carp) purchased from Jiangsu was temporarily raised in a 2000L aquarium after being tested for the absence of CyHV-2, equipped with a flow circulation fresh water treatment and purification system. After raising for 1 week, remove unhealthy individuals. Select healthy crucian carp with a weight of 350±50g and randomly group them into 200L experimental aquariums, 30 in each aquarium, change the water twice a day in the experimental aquarium, and change the water volume to 1 / 3, maintain the water temperature at 20±2°C, and continue to raise temporarily 2 weeks.
[0046] Preparation of pathogenic bacteria suspension: culture Aeromonas hydrophila to OD in LB liquid medium 600 was 0.6, then centrifuged at 5000rpm for 10 minutes at room temperature, collected the prec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com