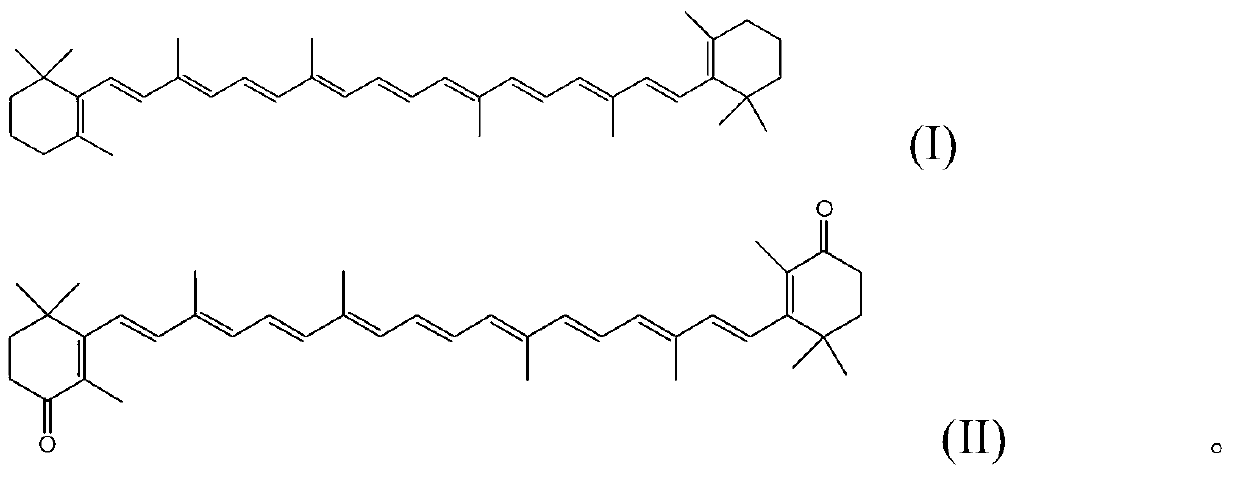
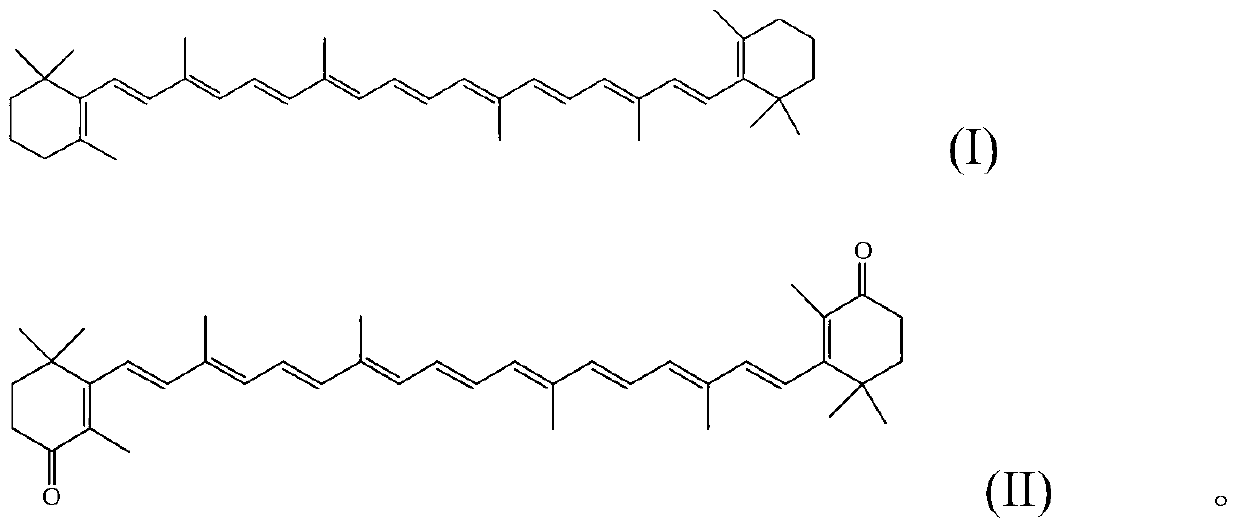
Method for preparing canthaxanthin by utilizing oxidized beta-carotene
A technology of carotene and canthaxanthin, which is applied in the direction of organic chemistry, can solve the problems that are difficult to apply to industrialization, low yield, unstable hypobromous acid, etc., and achieve low cost, simple operation, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 2.68g (5mmol) of β-carotene in a 250ml flask, add 50ml of dichloromethane, stir at room temperature, then take 3.21g (15mmol) of sodium periodate and dissolve it in 50ml of water, add it dropwise to the flask, and add catalyst iodine Sodium chloride 0.08g (0.5mmol), reacted for 3 hours, separated the organic phase, washed 3 times with water, then dried with anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain a crude product, which was recrystallized with absolute ethanol to obtain Purple-red cantharidin 1.50g, yield 53.2%. m.p.206℃-208℃; 1 H NMR (500M, δ, CDCl 3 ):6.66(dt,J=20.2Hz,7.7Hz,4H),6.30(ddd,J=36.0Hz,27.3Hz,15.6Hz,l0H),2.44-2.55(m,4H),1.95-2.06(m, 12H), 1.80-1.89 (m, 10H), 1.18 (s, 12H).
Embodiment 2
[0025] Take 2.68g (5mmol) of β-carotene in a 250ml flask, add 50ml of dichloromethane, stir at room temperature, then take 2.12g (10mmol) of sodium periodate and dissolve it in 30ml of water, add it dropwise to the flask, and add catalyst iodine Sodium chloride 0.08g (0.5mmol), reacted for 3 hours, separated the organic phase, washed 3 times with water, then dried with anhydrous sodium sulfate, filtered, the filtrate was spin-dried, and then recrystallized with absolute ethanol to obtain purple-red cantharidin 1.12 g, yield 39.7%.
Embodiment 3
[0027] Take 2.68g (5mmol) of β-carotene in a 250ml flask, add 50ml of dichloromethane, stir at room temperature, then take 4.27g (20mmol) of sodium periodate and dissolve it in 70ml of water, add it dropwise to the flask, and add catalyst iodine Sodium chloride 0.08g (0.5mmol), reacted for 3 hours, separated the organic phase, washed 3 times with water, then dried with anhydrous sodium sulfate, filtered, the filtrate was spin-dried, and then recrystallized with absolute ethanol to obtain purple-red cantharidin 1.56 g, yield 55.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com