Ruthenium complex and preparation method thereof and purpose of product as histone deacetylase inhibitor
A technology of ruthenium complexes and compounds, applied in the field of ruthenium complexes with excellent anti-tumor activity and its preparation, can solve the problems of histone hypoacetylation and achieve good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
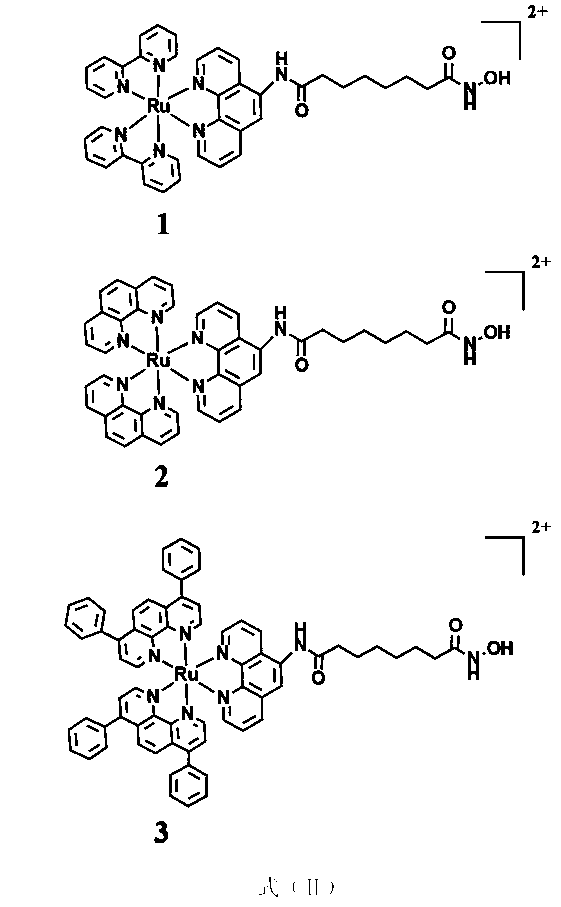
[0054] (1) Preparation of ligands:
[0055] Add 5-amino-1,10-phenanthroline (0.629 g, 3.226 mmol) and methyl 8-chloro-8-oxooctanoic acid (1 g, 4.839 mmol) into the reaction flask, and add catalytically metered DMAP , then stirred at room temperature in 45 ml of DMF solvent for 12 h, then rotary evaporated to remove most of the DMF, added a large amount of ether, filtered to obtain a light yellow solid, then added 50% aqueous hydroxylamine solution (the amount of the substance added to the aqueous hydroxylamine solution was light yellow 20 times that of the solid), methanol as the solvent (4 mL), add 1N NaOH solution (2 mL) and stir at room temperature for 30 min, adjust the pH to neutral with 1N HCl, you can see a light yellow precipitate formed, filter The ligand was obtained with a yield of 40%.
[0056] Elemental Analysis C 20 h 22 N 4 o 3 (Molecular weight is 366.4), theoretical value: C 65.56%, H 6.05%, N 15.29%; experimental value: C 65.17%, H 6.01%; N 14.96%. ESI-...
Embodiment 2
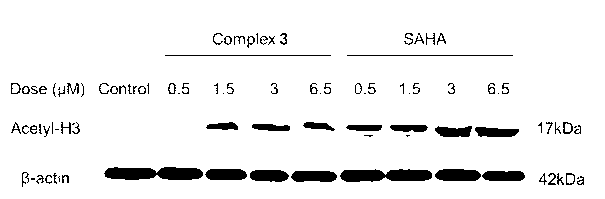
[0066] Example 2 Inhibition test of Ru(Ⅱ) complexes on histone deacetylase
[0067] HDAC enzyme activity test: the ability of ruthenium complexes to inhibit histone deacetylase is mainly detected by a fluorescent HDAC activity assay kit. The operation steps are roughly as follows. Add 0.5 μL of HeLa nuclear extract to a 96-well culture plate , then add HDAC substrate, 100 μL HDAC assay buffer, the negative control is without HeLa nuclear extract and HDAC substrate, and the wells where 1 μM Trichostatin A is added are set as positive control. Incubate the above reaction mixture in a 37°C incubator for 30 min, then add 10 μL of lysine developer to terminate the reaction, continue to incubate in a 37°C incubator for 30 min, take it out, and place the plate on a multi-functional microplate reader for determination For fluorescence values, the excitation wavelength was set to 340 nm and the emission wavelength was set to 460 nm. Histone deacetylase activity is expressed as relativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com