A kind of preparation method of sulfurized reforming catalyst
A technology for reforming catalysts and sulfide states, which is applied in catalyst activation/preparation, physical/chemical process catalysts, naphtha catalytic reforming, etc. It can solve the problems of fast deactivation rate, catalyst carbon deposition, poor stability, etc., to achieve Simplified reduction and presulfurization steps, improved reactivity, effects of high sulfur content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] (1) Preparation of oxidation state catalyst
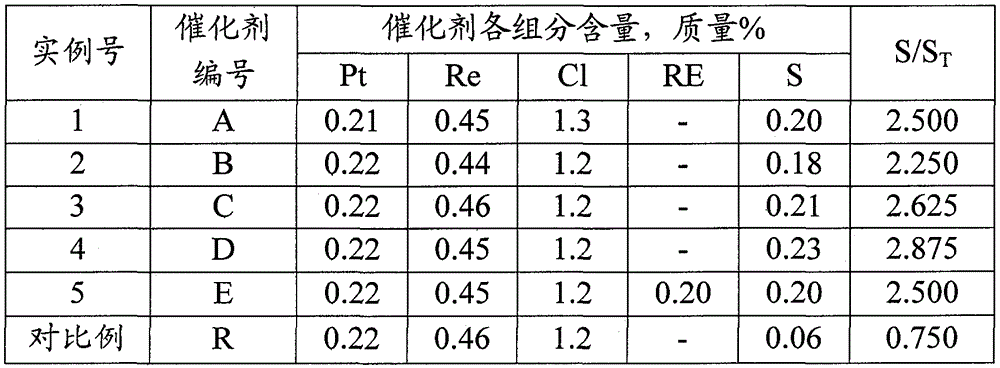
[0024] Take 50 grams of γ-Al 2 o 3The carrier is prepared with chloroplatinic acid, perrhenic acid and hydrochloric acid to form an impregnation solution, so that the liquid / solid volume ratio is 1.3, and the impregnation solution contains 0.22 mass % of platinum, 0.46 mass % of rhenium and 1.5 mass % of chlorine (all relative to dry basis Aluminum oxide calculation). First place the carrier in a reduced-pressure environment, make the pressure reach 0.02MPa, and maintain it for 0.5 hours, stop the decompression operation, introduce the impregnating solution, rotate and impregnate at 30°C for 3 hours, and then spin-dry at 60°C and 0.02MPa for 1 hour. The solid was taken out and dried at 120°C for 12 hours, then calcined with dry air at 500°C and the gas / solid volume ratio was 700 for 4 hours to obtain an oxidized catalyst.
[0025] (2) Preparation of sulfurized catalyst
[0026] Put the catalyst in the oxidized state into...
example 2
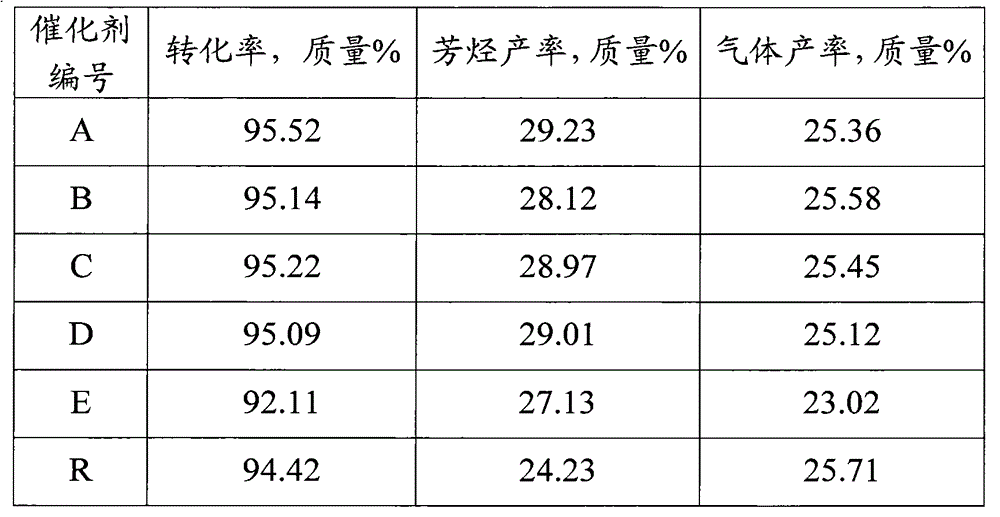
[0028] The method for preparing sulfided state catalyst by the example 1, difference is (2) step feeds the hydrogen that hydrogen sulfide content is 0.1mL / L in the reactor, make gas / solid volume ratio be 1000, press the speed of 30 ℃ / hour The temperature of the catalyst bed was raised to 460° C. and kept at this temperature for 2 hours. Then the temperature of the catalyst bed was lowered to 25° C., changed to nitrogen, purged for 0.5 hour, and the catalyst was unloaded and sealed for storage. The composition of the prepared sulfided catalyst B is shown in Table 1.
example 3
[0030] The method for preparing sulfide state catalyst by example 1, difference is (2) step feeds the hydrogen that hydrogen sulfide content is 2mL / L in the reactor, make gas / solid volume ratio be 600, by the rate of 50 DEG C / hour The temperature of the catalyst bed was raised to 460° C. and kept at this temperature for 2 hours. Then the temperature of the catalyst bed was lowered to 25° C., changed to nitrogen, purged for 0.5 hour, and the catalyst was unloaded and sealed for storage. The composition of the obtained sulfided catalyst C is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com