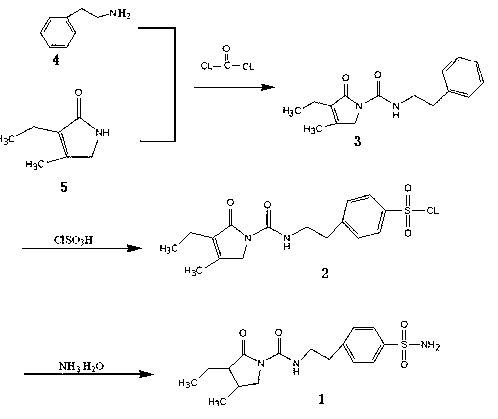
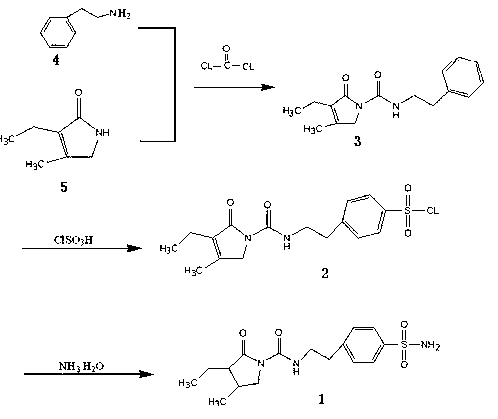
Method for synthesizing intermediate benzene sulfonamide of type 2 diabetes mellitus drug glimepiride
A technology for isobenzenesulfonamide and diabetes drugs, which is applied in the field of compound preparation, can solve the problems of complex process steps and inconvenient operation, and achieve the effect of simple process and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 1200 ml of dichloromethane, 170 g of phenethylamine, and 175 g of 3-ethyl-4-methylpyrrolidinone into a 3000 ml three-necked flask, and control the temperature at -10 to 0°C to feed in metered phosgene 132 gram, passed through in about 2 hours, continued to react for 3 hours after passing through, then warmed up to room temperature and stirred for 2 hours to the end of the reaction, added 800 milliliters of water and stirred, concentrated to remove solvent methylene chloride, then added 1000 milliliters of water, stirred and cooled to 0°C, and stirred at this temperature for 3 hours, filtered, washed with 100 milliliters of ethanol, and dried to obtain 195 grams of intermediate N-[2-(3-ethyl-4-methyl-2-oxidized-3-pyrroline -1-Carboxamido)ethyl]-benzene.
[0015] Pump 640 grams of chlorosulfonic acid into a 2000 milliliter reactor, put the jacket into the ice brine and cool down to about 10°C, keep the internal temperature at 10-15°C, and add 195 grams of the inte...
Embodiment 2
[0017] Add 1200 ml of dichloromethane, 170 g of phenethylamine, and 175 g of 3-ethyl-4-methylpyrrolidinone into a 3000 ml three-necked flask, and control the temperature from -10 to 0 ° C to feed metered phosgene 152 gram, passed through in about 2 hours, continued to react for 3 hours after passing through, then warmed up to room temperature and stirred for 2 hours to the end of the reaction, added 800 milliliters of water and stirred, concentrated to remove solvent dichloromethane, then added 1000 milliliters of water, stirred and cooled to 0 ℃, and stirred at this temperature for 3 hours, filtered, washed with 100 milliliters of ethanol, and dried to obtain 198 grams of intermediate N-[2-(3-ethyl-4-methyl-2-oxidized-3-pyrroline- 1-formamido)ethyl]-benzene.
[0018] Pump 640 grams of chlorosulfonic acid into a 2000 milliliter reactor, put the jacket into ice brine to cool down to about 10°C, keep the internal temperature at 10-15°C, and add 198 grams of the intermediate N-[2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com