Method for splitting optically pure 2-octyl alcohol by adopting enzyme catalysis
A technology of octanol and octanol ester, which is applied in the field of R-2-octanol, can solve the problems of low space-time yield, loss of economic feasibility, low loading ratio of substrate and enzyme, and achieve low cost, easy separation, and easy The effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
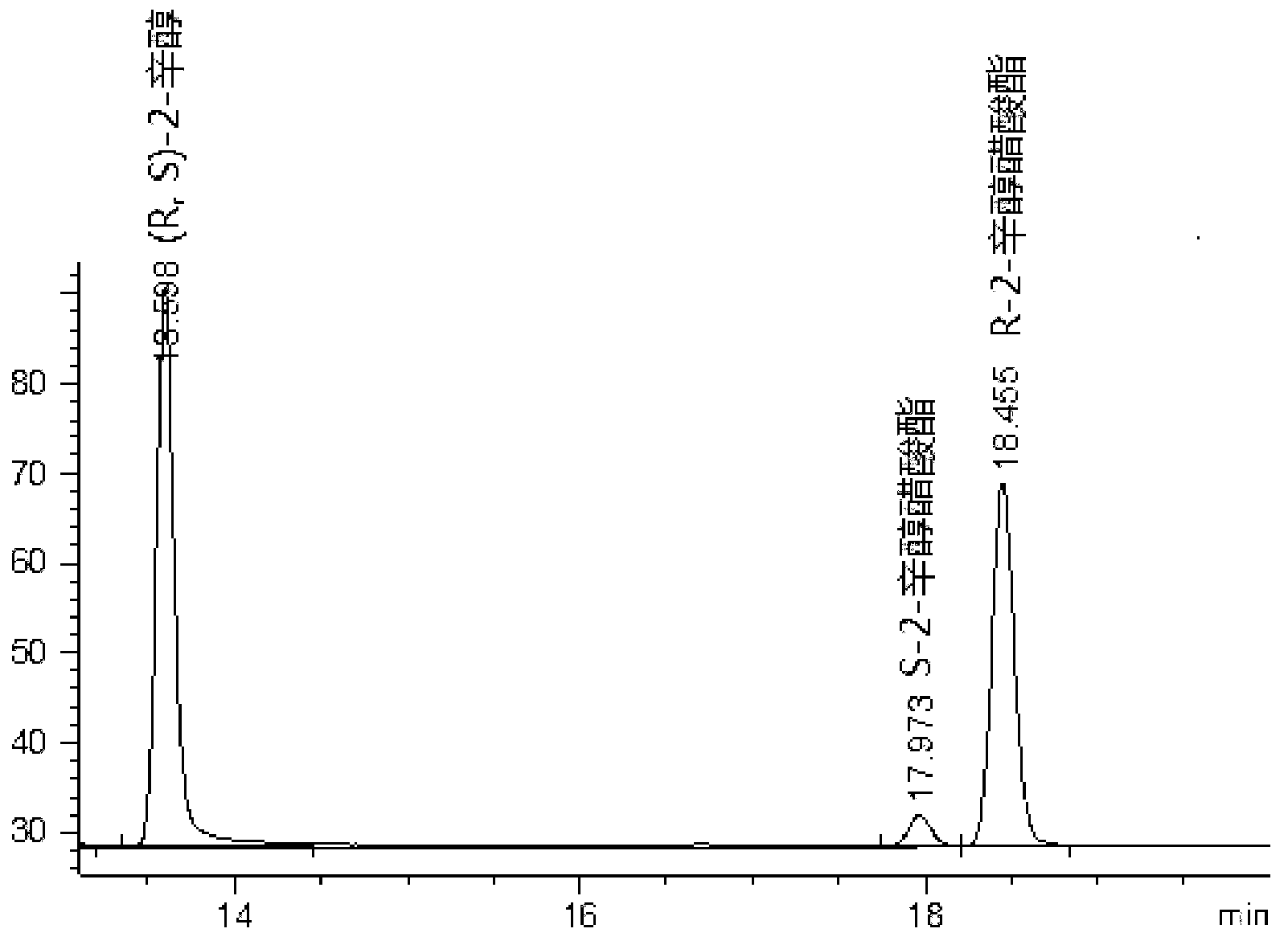
[0034] In a 25ml Erlenmeyer flask with a stopper, add 10ml of n-hexane, 1mmol of racemic 2-octanol (purchased from Sigma, the same below), 2mmol of vinyl acetate, and 0.1g of immobilized enzyme immobilized by macroporous resin. And 10μL of 0.1mol / L phosphate buffer with pH=8. The reaction mixture was placed on a shaker at 30° C., and was shaken and reacted at a constant temperature at a speed of 150 rpm for 20 hours. 1 microliter of the reaction mixture was taken for gas chromatography analysis. The analysis method was as follows:
[0035] The chiral column was G-TA Capillary Column (40m×0.25mm×0.12μm, Astech, USA), and the temperature of the column oven, injection port and detector were 110°C, 200°C and 250°C, respectively. Nitrogen was used as the carrier gas with a flow rate of 40 mL / min and a split ratio of 1:20.
[0036] The above samples were analyzed and the results were as follows: figure 1 Shown, YLL lipase preferentially catalyzes the R-isomer, and reaction 20 hour...
Embodiment 2
[0043] In a 25ml Erlenmeyer flask with a stopper, add 10ml of n-hexane, 1mmol of racemic 2-octanol, 2mmol of succinic anhydride, 0.1g of immobilized enzyme immobilized by macroporous resin and 10μL of 0.1mol / L pH=8 Phosphate buffer. The reaction mixture was placed on a shaker at 30°C, and was shaken and reacted at a constant temperature at 150 rpm for 20 hours. Take 1 microliter of the reaction mixture and detect it under the same conditions as in Example 1. The result is the conversion rate c is 0.51, and the stereoselectivity of the enzyme is E=150.
[0044] The above reaction mixture was extracted with an equal volume of 1 mol / L sodium carbonate solution to obtain S-2-octanol and R-2-octanol succinate.
[0045] In order to further hydrolyze the obtained R-2-octanol succinate to generate R-2-octanol, an aqueous sodium hydroxide solution with pH=11 is used as a catalyst to catalyze the hydrolysis.
Embodiment 3
[0047]In a 25ml Erlenmeyer flask with a stopper, add 10ml of n-hexane, 1mmol of racemic 2-octanol, 2mmol of hexanoic acid, 0.1g of immobilized enzyme immobilized by macroporous resin and 20μL of 0.1mol / L pH=8 Phosphate buffered saline and 1 g of silica gel. The reaction mixture was placed on a shaker at 30°C, and was shaken and reacted at a constant temperature at 150 rpm for 20 hours. Take 1 microliter of the reaction mixture and detect it under the same conditions as in Example 1. The result is the conversion rate c is 0.43, and the stereoselectivity of the enzyme is E=135.
[0048] The above reaction mixture was passed through a silica gel chromatography column, eluted with a mixed solution of ethyl acetate and chloroform (v / v=1:1), and collected in sections to obtain S-2-octanol and R-2-octanol-hexyl ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com