Anti-allergic rhinitis combination drug
An anti-allergic and drug-based technology, applied in drug combinations, allergic diseases, medical formulas, etc., can solve the problems of incurability, poor treatment effect, high cost, etc., and achieve the disappearance of runny nose symptoms, not easy to relapse, and short course of treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 (medicine combination): vitamin B6 sheet (2 * specification 10mg=20mg); Chlorpheniramine maleate sheet (2 * specification 4mg=8mg); Dexamethasone acetate sheet (2 * specification 0.75mg = 1.5mg); vitamin D2 calcium hydrogen phosphate tablets 2 pieces × specifications 150mg = calcium hydrogen phosphate 300mg (that is, vitamin D2 calcium hydrogen phosphate tablets, each tablet contains calcium hydrogen phosphate 150mg, vitamin D2500 units); bee honeycomb 50mg. Crush vitamin B6 tablets, chlorpheniramine maleate tablets, dexamethasone acetate tablets and vitamin D2 calcium hydrogen phosphate tablets into 150-200 mesh fine powder, mix it with dried and crushed 150-200 mesh honey honeycomb powder Capsules, wherein each capsule weighs about 700mg.
Embodiment 2
[0032] Embodiment 2 (medicine combination): vitamin B6 sheet (2 * specification 10mg=20mg); Chlorpheniramine maleate sheet (1 * specification 4mg=4mg); Dexamethasone acetate sheet (1 * specification 0.75mg = 0.75mg); vitamin D2 calcium hydrogen phosphate tablets 2 pieces × specification 150mg = calcium hydrogen phosphate 300mg (that is, vitamin D2 calcium hydrogen phosphate tablets, each tablet contains calcium hydrogen phosphate 150mg, vitamin D2500 units); bee honeycomb 30mg. Crush vitamin B6 tablets, chlorpheniramine maleate tablets, dexamethasone acetate tablets and vitamin D2 calcium hydrogen phosphate tablets into 150-200 mesh fine powder, mix it with dried and crushed 150-200 mesh honey honeycomb powder Capsules, wherein each capsule weighs about 550mg.
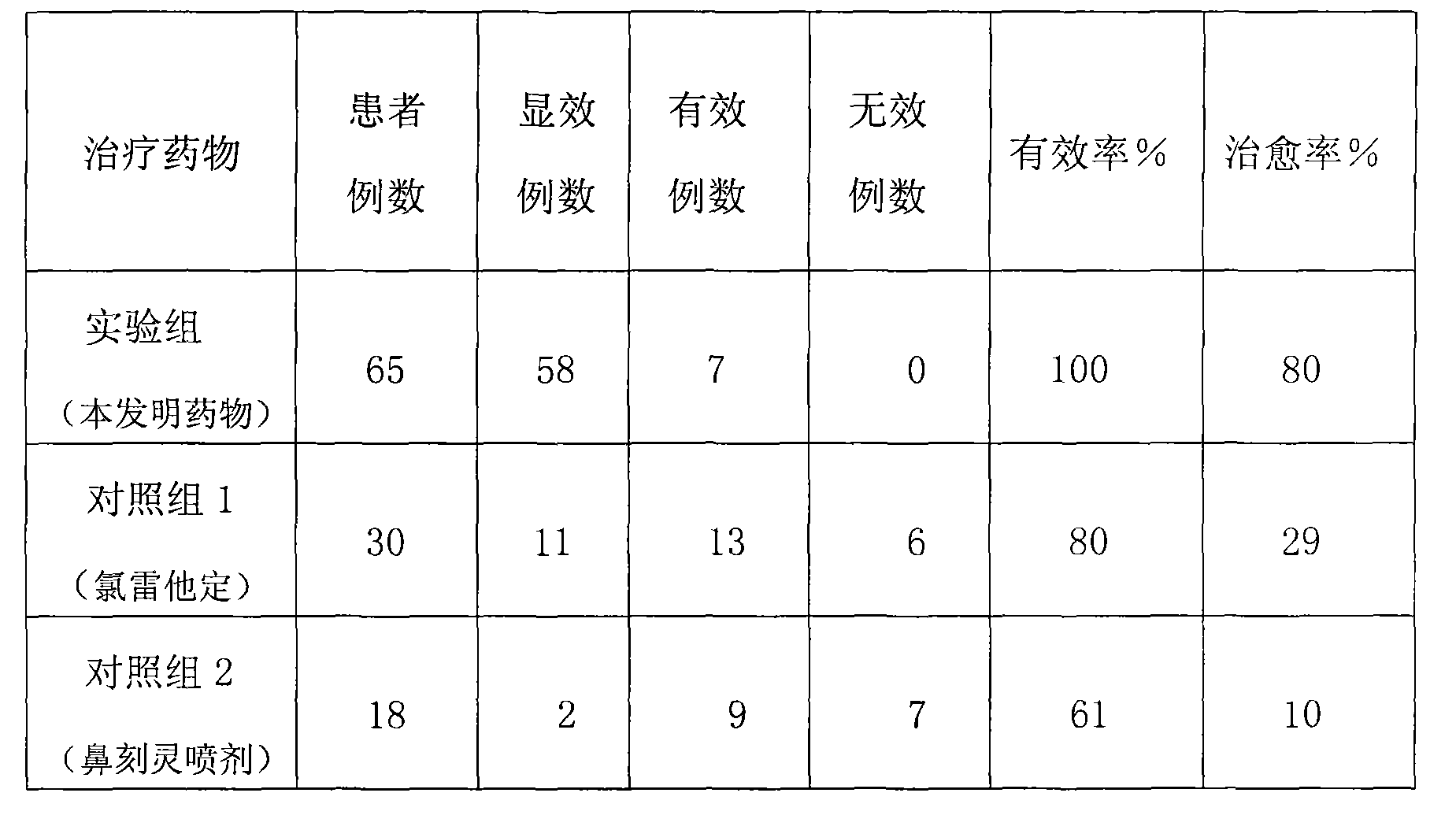
[0033] Typical cases are extracted as follows according to the observation of combination drug therapy of the present invention:
[0034] Case 1: A 57-year-old male patient suffered from allergic rhinitis in 1989 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com