Polyimide and coating composition formed thereby
一种聚酰亚胺、组合物的技术,应用在软性组件领域,能够解决易黄变、变脆、材料特性与操作性冲突等问题,达到不易黄变、优异耐化性与热性质、透明性佳的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0098] In the following examples, the abbreviations used are defined as follows:
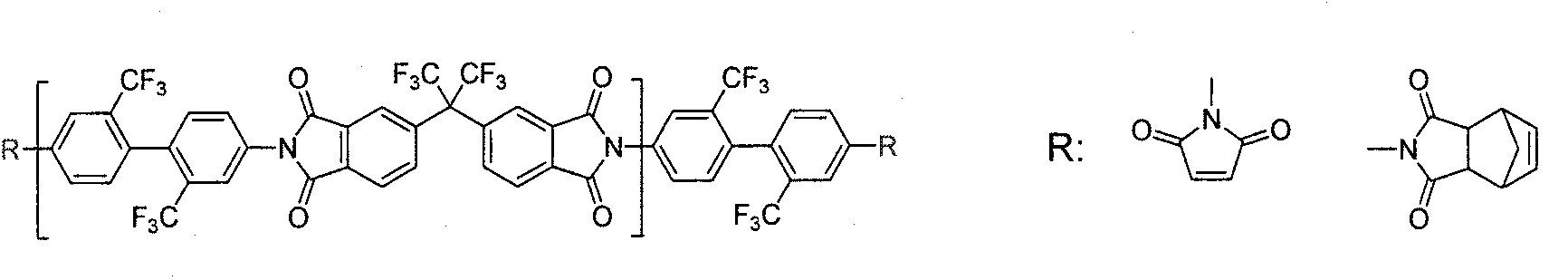
[0099] 6FDA: 4,4'-hexafluoroisopropylidene-2,2-bis(phthalic anhydride) (4,4'-hexafluoroisopropylidene-2,2-bis-(phthalic acid anhydride)
[0100] S-BPDA: 3,3',4,4'-biphenyltetracarboxylic dianhydride (3,3',4,4'-Biphenyltetracarboxylic dianhydride)
[0101] CBDA: 1,2,3,4-cyclobutanetetracarboxylic dianhydride (1,2,3,4-cyclobutanetetracarboxylic dianhydride)
[0102] BPAF: 5,5'-(9H-fluorene-9,9-diyl)diisobenzofuran-1,3-dione (5,5'-(9H-fluorene-9,9-diyl)diisobenzofuran- 1,3-dione)
[0103] TFMB: 2,2'-bis(trifluoromethyl)-p-diaminobiphenyl (2,2'-bis(trifluoromethyl)benzidine)
[0104] CHDA: 1,4-diaminocyclohexane (1,4-diaminocyclohexane)
[0105] BAFL: 4-(9-(4-aminophenyl)-9H-fluoren-9-yl)aniline (4-(9-(4-aminophenyl)-9H-fluoren-9-yl)benzonamine)
[0106] TMDA: 1-(4-aminophenyl)-2,3-dihydro-1,3,3-trimethyl-1 hydrogen-indene-5-amine (1-(4-aminophenyl)-2,3- dihydro-1,3,3-trimethyl-1H-inden-5-amin...
Embodiment A-1
[0115] Embodiment A-1: synthesis has the polyimide (PI-A1) of tetraacid terminal group
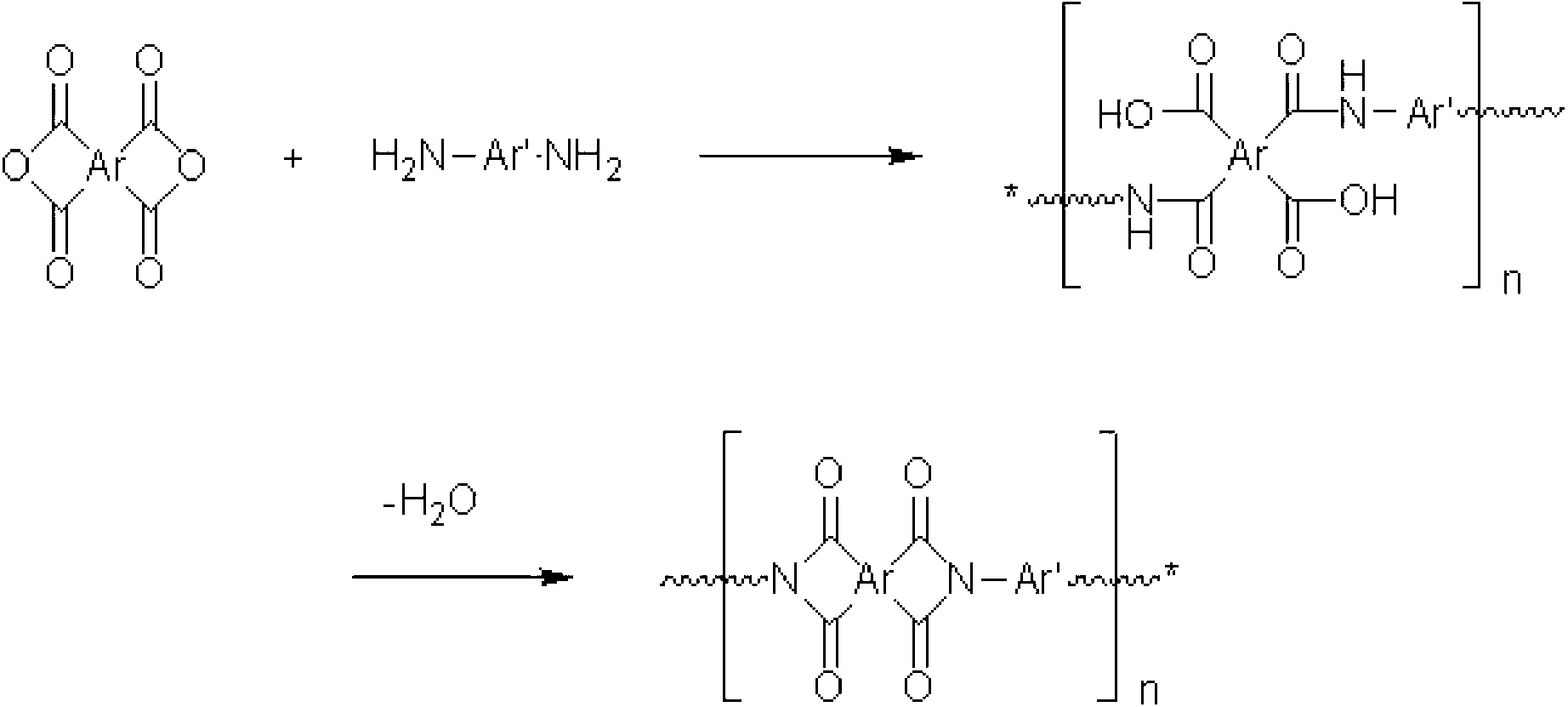
[0116]Weigh 97.7328g (0.22mol) of 6FDA and 64.046g (0.2mol) of TFMB, add 640mL of NMP, stir at room temperature for 1 hour, then raise the temperature to 60°C and stir for 4 hours. After 4 hours, 320 mL of xylene was added, and water was removed with a dean-stark apparatus at 180°C. After the water removal is complete, the solution is lowered to room temperature, and then 0.7208 g (0.04 mol) of H 2 O. After the addition was complete, the solution was warmed to 80°C and stirred for 4 hours. You can get PI-A1.
Embodiment A-2
[0117] Embodiment A-2: synthesis has the polyimide (PI-A2) of tetraacid terminal group
[0118] Weigh 43.1442g (0.22mol) of CBDA and 64.046g (0.2mol) of TFMB, add 640mL of NMP, stir at room temperature for 1 hour, then raise the temperature to 60°C and stir for 4 hours. After 4 hours, 320 mL of xylene was added, and water was removed with a dean-stark apparatus at 180°C. After the water removal is complete, the solution is lowered to room temperature, and then 0.7208 g (0.04 mol) of H 2 O. After the addition was complete, the solution was warmed to 80°C and stirred for 4 hours. You can get PI-A2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com