Preparation method of dichloropropanol
A production method and a technology for dichloropropanol, which are applied in the production field of dichloropropanol, can solve the problems of short service life of catalysts, equipment corrosion, organic chloride waste water, etc., and achieve mild reaction conditions, less side reactions, and reduced sewage discharge. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
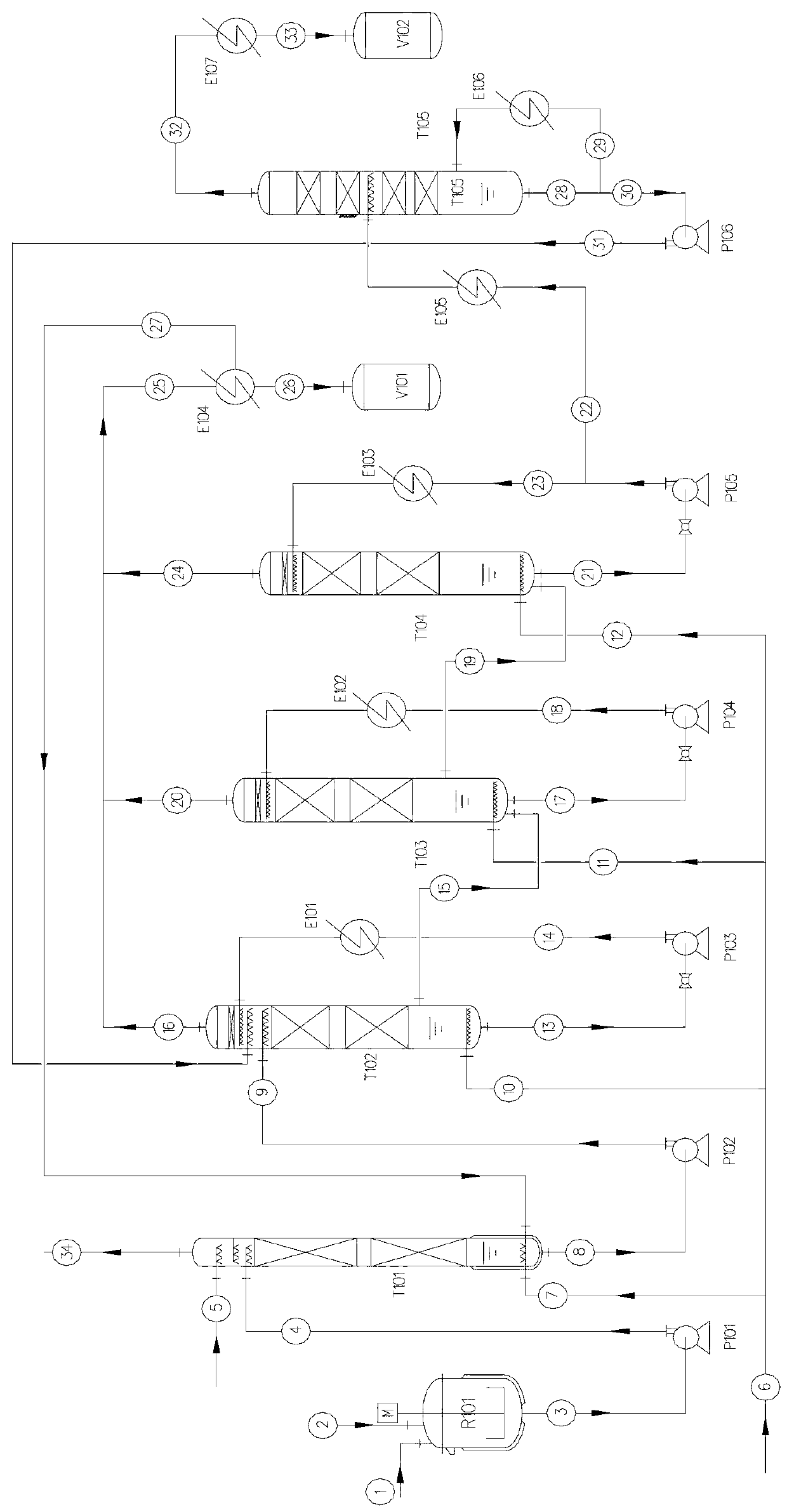
[0035] Embodiment 1: as figure 1 As shown, it is a 3-stage chlorination reactor series process, in which T102~T104 are tower chlorination reactors, T101 is a tail gas absorption tower, and T105 is a dichloropropanol (DCP) distillation tower.
[0036] Add glycerin ① and tin salt catalyst ② into the R101 kettle to dissolve into a homogeneous phase, and from the outlet of the kettle ③, through the pump P101, measure and transport ④ into the top of the T101 tower, and enter the tower through the distributor. The catalyst is added once, and under normal operating conditions, it is not added and recycled. The other part of glycerol (without catalyst) feed line ⑤ is directly fed from the top of T101 tower according to the set value of the production scale, and enters the tower through the distributor. Hydrogen chloride ⑦ from hydrogen chloride main pipe ⑥ enters the tower from the T101 tower kettle through the gas distributor, glycerin and hydrogen chloride contact and react counter...
Embodiment 2
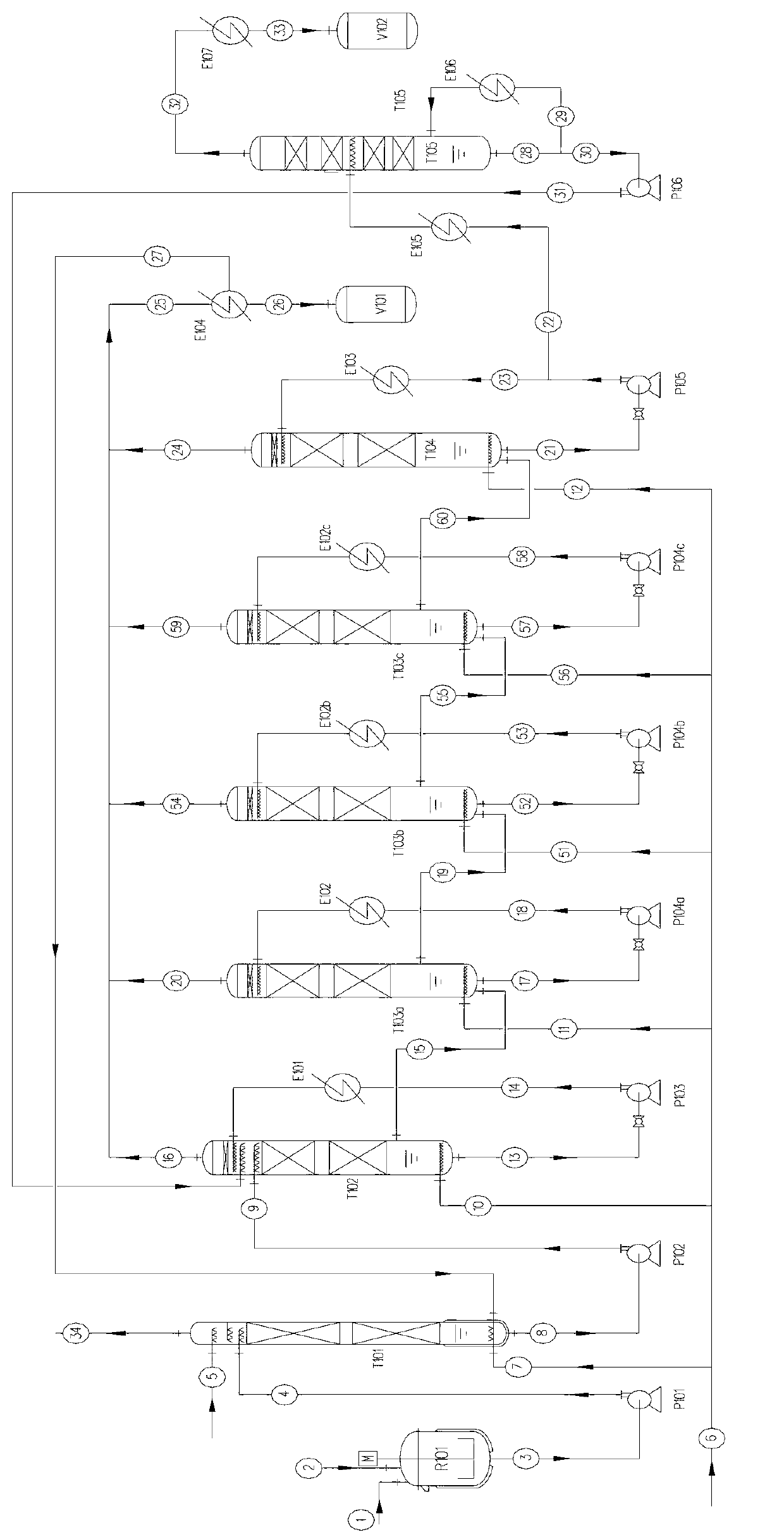
[0046] Embodiment 2: as figure 2 As shown, it is a 5-stage chlorination reactor series process, in which the chlorination reaction tower is composed of five towers T102, T103a, T103b, T103c and T104 connected in series, T101 is the tail gas absorption tower, T105 is dichloropropanol (DCP) refined Distillation tower.
[0047] 1) Operating temperature: 100°C,
[0048] 2) Operating pressure: 40kPa(G).
[0049] 3) Chlorination reaction time: 12 hours.
[0050] 4) Chlorination catalyst: tin compound salt, add 1%wt catalyst of the total mass of glycerin at one time when starting up for the first time, and recycle it in normal operating state without adding it.
[0051] Other conditions are with embodiment 1.
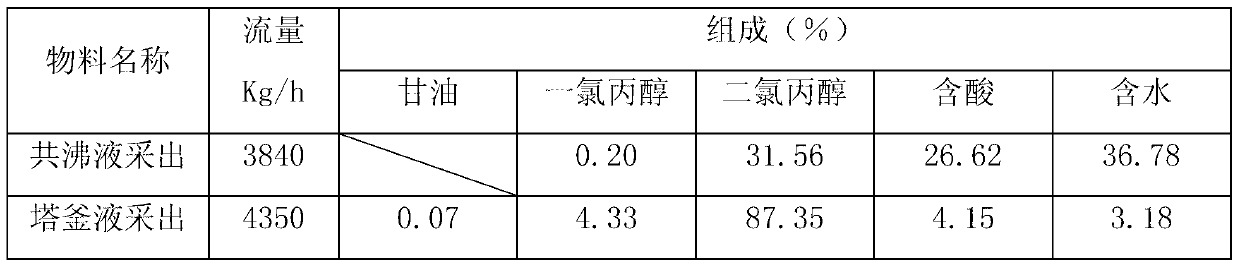
[0052] 1) The composition of the chlorinated liquid product is detected by gas chromatography as follows:
[0053]
Embodiment 3
[0054] Embodiment 3: as figure 1 As shown, it is a 3-stage chlorination reactor series process, the chlorination reaction temperature is 115°C, the reaction time is 10 hours, the chlorination catalyst is tin compound salt, and 7%wt catalyst of the total mass of glycerin is added at one time when starting up for the first time, and the normal operation The state is recycled and no longer added. Other conditions are with embodiment 1.
[0055] Detect the chlorinated liquid product components by gas chromatography as follows:
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com