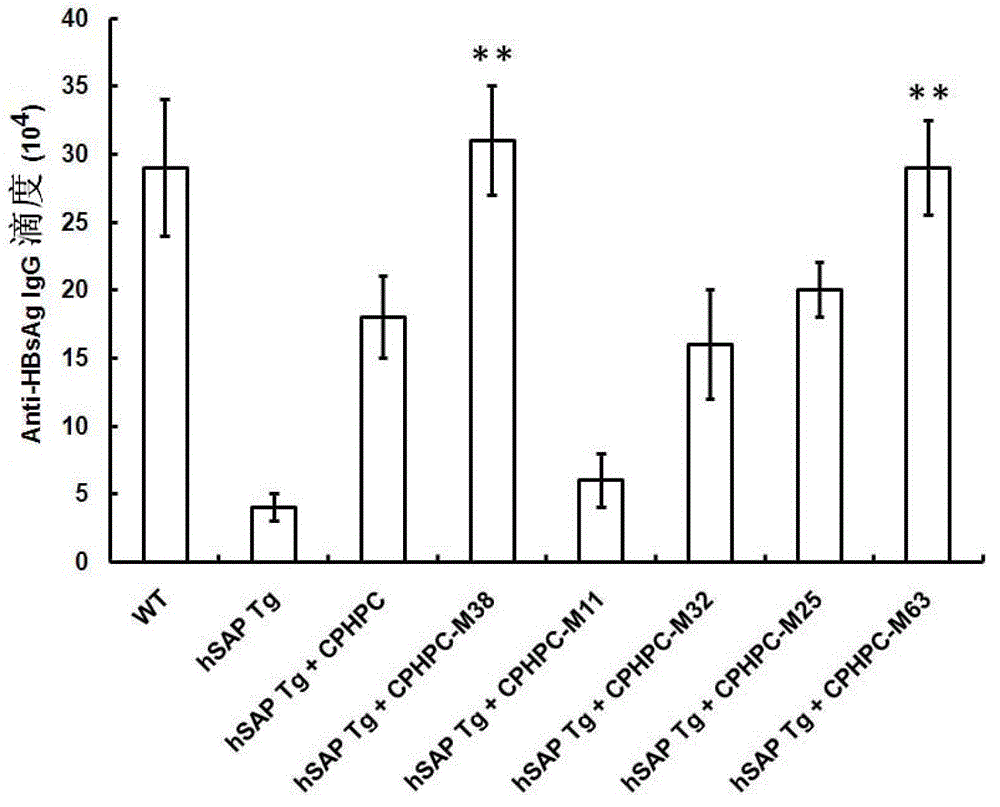
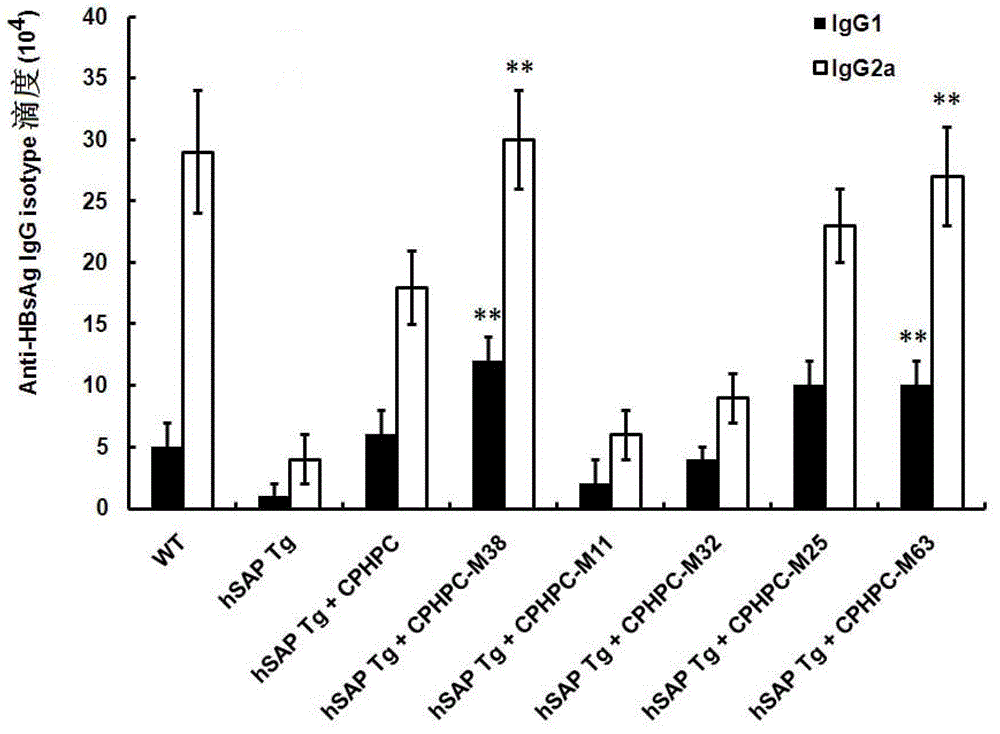
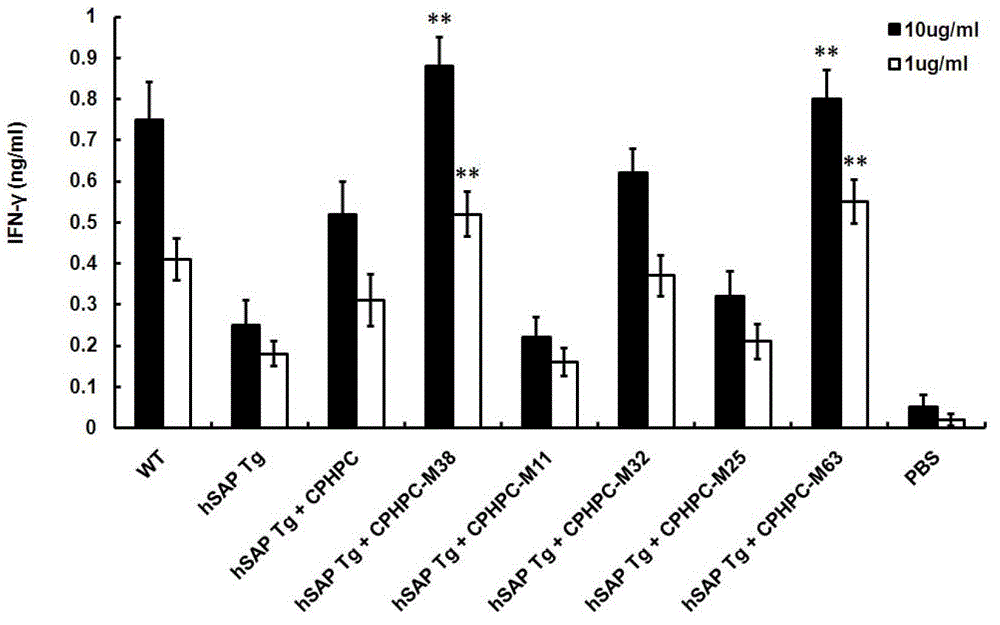
Adipamide D-hydroxyproline derivative and application thereof as nucleic acid vaccine adjuvant
A technology of hydroxyproline and adipoyl bisulfite, applied in the field of medicine, can solve the problems of decreased immune response and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of 1-[6-[4R-hydroxy-2R-carboxy-1-pyrrolidinyl]-6-oxohexanoyl]-pyrrolidine-4R-hydroxy-2R-carboxylic acid (1)
[0041]2.9g (20.0mmol) of 4R-hydroxy-cis-D-proline methyl ester hydrochloride was dissolved in 100mL of anhydrous dichloromethane, and 3.5mL of diisopropylethylamine (DIPEA) was added at 0°C. 1.46g (10.0mmol) adipic acid, 3.4g (25.2mmol) N-hydroxybenzotriazole (HOBt) and 4.8g (25.6mmol) 1-ethyl-3-(3-dimethylaminopropyl) Carbodiimide hydrochloride (EDC·HCl), stirred for 24 hours, recovered the solvent under reduced pressure, added 100mL of ethyl acetate and 50mL of 10% (w / v) citric acid aqueous solution to the oily residue, shaken fully, and separated the acetic acid The ethyl ester layer was washed successively with saturated aqueous sodium carbonate, water, and saturated brine, and anhydrous Na 2 SO 4 Dry, recover the solvent, add 80mL of methanol and 80mL of 0.5mol / L sodium hydroxide aqueous solution, stir for 2h, adjust the pH to 5-6 w...
Embodiment 2
[0044] Example 2: Preparation of 1-[6-[4R-hydroxy-2R-carboxy-1-pyrrolidinyl]-6-oxohexanoyl]-pyrrolidine-4S-hydroxy-2R-carboxylic acid (3)
[0045] 1.45g (10.0mmol) of 4R-hydroxy-cis-D-proline methyl ester hydrochloride was dissolved in 50mL of anhydrous dichloromethane, and 2.0mL of DIPEA and 1.28g (10.0mmol) of adipic anhydride were added at 0°C , stirred for 24 hours, then added 1.45g (10.0mmol) of 4S-hydroxy-trans-D-proline methyl ester hydrochloride, 1.5mL DIPEA1.7g (12.6mmol) HOBt and 2.4g (12.8mmol) EDC·HCl , continue to stir and react for 24 hours, recover the solvent under reduced pressure, add 100mL ethyl acetate and 50mL10% (w / v) citric acid aqueous solution to the oily residue, shake fully, separate the ethyl acetate layer, and successively wash with saturated sodium carbonate water Liquid, water, saturated brine washing, anhydrous Na 2 SO 4 Dry, recover the solvent, add 80mL of methanol and 80mL of 0.5mol / L sodium hydroxide aqueous solution, stir for 2h, adjust t...
Embodiment 3
[0049] Example 3: 1-[6-[4R-azido-2R-carboxy-1-pyrrolidinyl]-6-oxohexanoyl]-pyrrolidine-4S-azido-2R-carboxylic acid (6 ) preparation
[0050] 1.70g (10.0mmol) of 4R-azido-cis-D-proline methyl ester hydrochloride was dissolved in 50mL of anhydrous dichloromethane, and 2.0mL of DIPEA and 1.28g (10.0mmol) of hexyl Diacid anhydride, stirred and reacted for 24h, then added 1.70g (10.0mmol) of 4S-azido-trans-D-proline methyl ester hydrochloride, 1.5mL DIPEA1.7g (12.6mmol) HOBt and 2.4g (12.8 mmol) EDC·HCl, continue to stir and react for 24 hours, recover the solvent under reduced pressure, add 100mL ethyl acetate and 50mL 10% (w / v) citric acid aqueous solution to the oily residue, shake fully, separate the ethyl acetate layer, and successively Wash with saturated sodium carbonate aqueous solution, water, saturated brine, anhydrous Na 2 SO 4 Dry, recover the solvent, add 80mL of methanol and 80mL of 0.5mol / L sodium hydroxide aqueous solution, stir for 2h, adjust the pH to 5-6 with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com