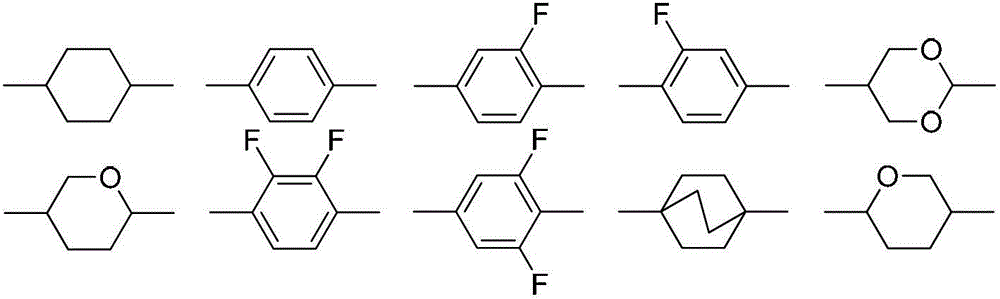
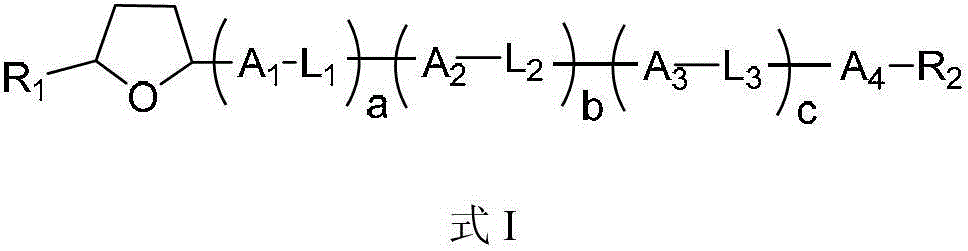Liquid crystal compounds containing tetrahydrofuran structure
A compound, hydrogen atom technology, applied in organic chemistry, liquid crystal materials, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
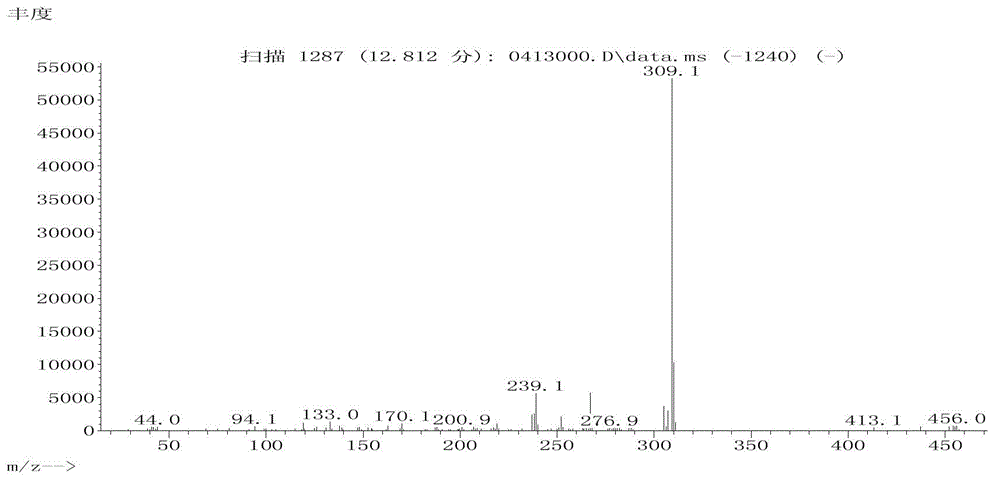
Image
Examples
Embodiment 1
[0142] Embodiment 1, compound shown in preparation formula I
[0143]
[0144] step 1:
[0145]
[0146]Add 46.3g (0.24mol) of 3,5-difluorobromobenzene (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. 2.5N) n-butyllithium (reactant), keep warm for 1 hour after dropping, at the same temperature, add dropwise a mixed solution of 0.216mol1,4-butyrolactone (reactant) and 50ml dry tetrahydrofuran (solvent), stir after dropping After 30 minutes, the temperature was raised naturally, and 200ml of saturated ammonium chloride aqueous solution was added dropwise at about 0°C (to adjust the pH value), separated, the aqueous phase was extracted with 200ml of ethyl acetate (solvent), the organic phase was washed with water, and spin-dried to obtain 43g (GC: 89%) liquid, in another 1L there-necked bottle, add 50g of the product obtained above, 500ml of dry dichloromethane (solvent), under nitrogen protection, cool to -25~-20°C, add dropwise 63.3ml (...
Embodiment 2
[0165] Embodiment 2, compound shown in preparation formula I
[0166]
[0167] step 1:
[0168]
[0169] Add 56.64g (0.24mol) of 1,4-dibromobenzene (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. N) n-butyllithium (reactant), dropwise insulation 1 hour, under the same temperature, drop into the mixed solution of 0.216mol1,4-butyrolactone (reactant) and 50ml dry tetrahydrofuran (solvent), dropwise and stir for 30 Minutes, heat up naturally, drop 200ml of saturated ammonium chloride aqueous solution (adjust the pH value) at about 0°C, separate the liquids, extract the aqueous phase with 200ml ethyl acetate (solvent), wash the organic phase with water, and spin dry to obtain 50g (GC: 89 %) liquid, in another 1L there-necked bottle, add 50g of the product obtained above, 500ml of dry dichloromethane (solvent), under nitrogen protection, cool to -25~-20°C, add dropwise 63.3ml (0.397mol, 2.2eq) Triethylsilylhydrogen (reactant), after dropp...
Embodiment 3
[0191] Embodiment 3, compound shown in preparation formula I
[0192]
[0193] step 1
[0194]
[0195] Add 0.1mol (1-a) in the reaction flask, 0.12mol m-fluorophenylboronic acid (reactant), 0.2mol sodium carbonate (reactant), 80ml toluene (solvent), 60ml ethanol (solvent), 60ml water (solvent), Under the protection of nitrogen, 0.4 g of tetrakis(triphenylphosphine)palladium (catalyst) was added, stirred and heated to reflux for 3 hours. Cool down to room temperature, separate the layers, extract the aqueous phase with 50ml of toluene (solvent), and wash the organic phase with water until neutral. Evaporate the solvent to dryness, dissolve the resultant in 100ml toluene, decolorize it through a silica gel column, elute with toluene (solvent), collect the eluate and evaporate the solvent to dryness, dissolve with 3 times of petroleum ether, freeze and recrystallize at -20°C, and filter with suction. White crystals (3-a) were obtained. The yield is 90%, and the gas chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com