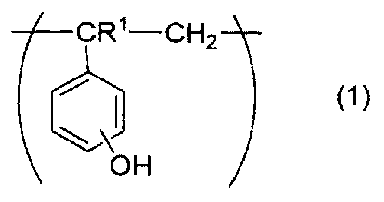
Metallic nanoparticle composite and method for producing the same
A technology of metal nanoparticles and manufacturing methods, applied in cable/conductor manufacturing, nanotechnology, nanotechnology, etc., can solve the problems of frequent replacement of membranes, high cost, membrane clogging, etc., achieve simple and easy manufacturing, and maintain stability Excellent, excellent dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Example 1 Manufacture of gold nanoparticle dispersion (1)
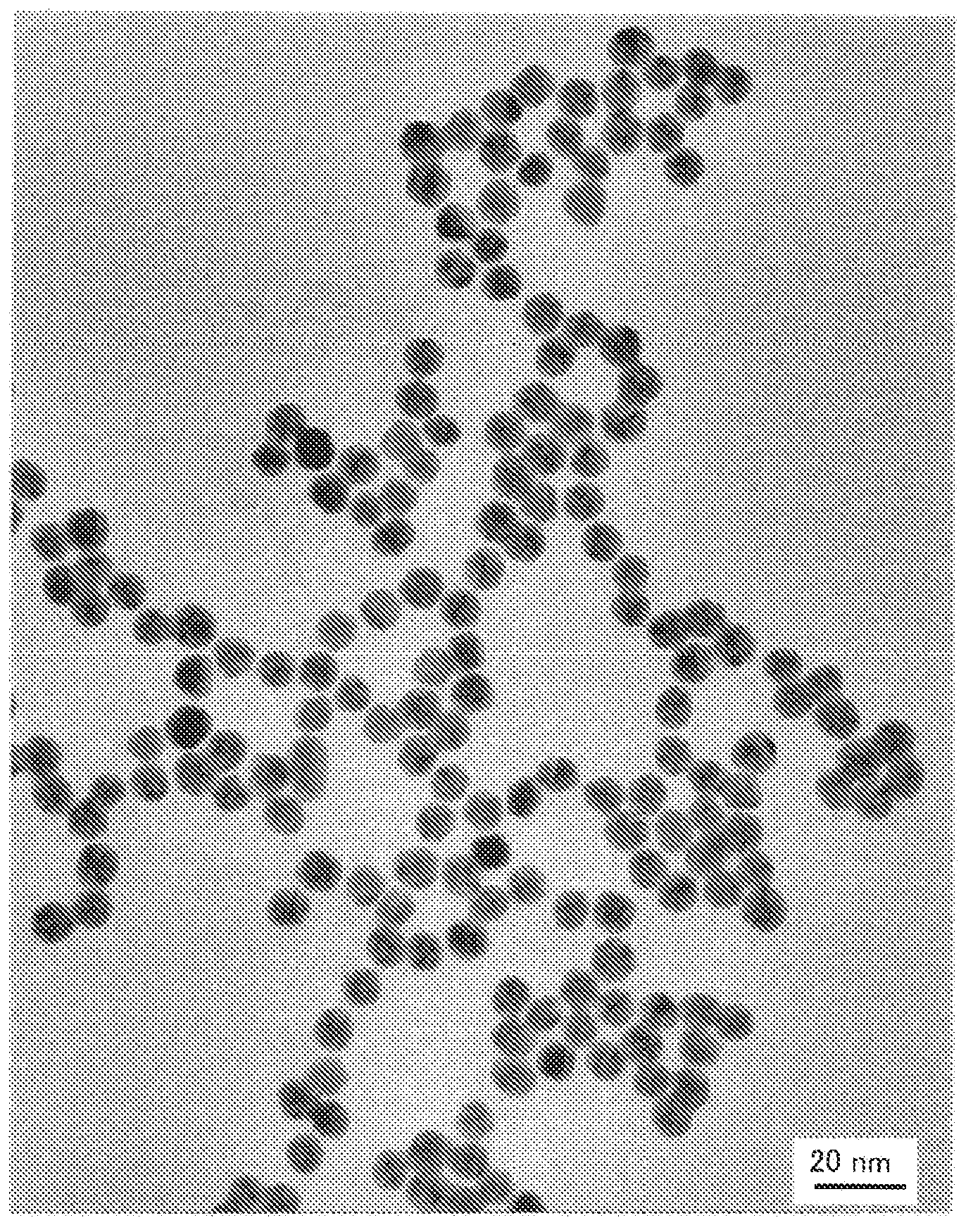
[0186] Into a 100 mL glass eggplant-shaped flask, 27 mg of the star polymer A obtained in Synthesis Example 1, 1.8 mL of an aqueous chloroauric acid solution with a metal content of 1 mol / L (354 mg of gold) and 90 mL of ion-exchanged water were charged and stirred. Then, in the state of maintaining stirring, add NaBH which is 10 molar equivalents to chloroauric acid 4 , performing a reduction reaction at room temperature for 1 hour to obtain a gold nanoparticle dispersion containing a gold nanoparticle complex.
[0187] The dispersion liquid was red, and the absorption spectrum of the dispersion liquid was measured. As a result, plasmon absorption from gold nanoparticles was observed around 540 nm, and it was considered that gold nanoparticles were generated.
[0188] In addition, observation of the above-mentioned dispersion liquid using TEM confirmed that the particle diameter of the generated gold nanopar...
Embodiment 2
[0192] Manufacture of Embodiment 2 Silver Nanoparticle Dispersion Liquid (1)
[0193] Into a 100 mL glass eggplant-shaped flask, 23 mg of the star polymer A obtained in Synthesis Example 1, 7.85 μL of an aqueous silver nitrate solution with a metal content of 1 mol / L (0.85 mg of silver) and 74 mL of ion-exchanged water were charged and stirred. Next, 10 molar equivalents of NaBH to silver nitrate were added while stirring. 4 , the reduction reaction was carried out at room temperature for 1 hour to obtain a silver nanoparticle dispersion containing a silver nanoparticle complex.
[0194] The above-mentioned dispersion liquid was yellow, and the absorption spectrum of the dispersion liquid was measured. As a result, plasmon absorption derived from silver nanoparticles was observed around 395 nm.
Embodiment 3
[0195] Manufacture of Example 3 Gold Nanoparticle Dispersion (2)
[0196] Into a 100 mL glass eggplant-shaped flask, 27 mg of the star-shaped polymer B obtained in Synthesis Example 2, 1.8 mL of an aqueous chloroauric acid solution with a metal content of 1 mol / L (354 mg of gold), and 90 mL of ion-exchanged water were charged and stirred. Then, in the state of maintaining stirring, add NaBH which is 10 molar equivalents to chloroauric acid 4 , performing a reduction reaction at room temperature for 1 hour to obtain a gold nanoparticle dispersion containing a gold nanoparticle complex.
[0197] The above-mentioned dispersion liquid was red, and the absorption spectrum of the dispersion liquid was measured. As a result, plasmon absorption due to gold nanoparticles was observed around 535 nm, and it was considered that gold nanoparticles were generated.
[0198] Comparative Example 1 Production of Gold Nanoparticle Dispersion Liquid (3)
[0199] A gold nanoparticle dispersi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com