Tamoxifen citrate sustained-release tablets
A technology of tamoxifen and sustained-release tablets, applied in the field of sustained-release tablets, can solve the problems of low bioavailability, prolonging biological half-life, slowing down absorption rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
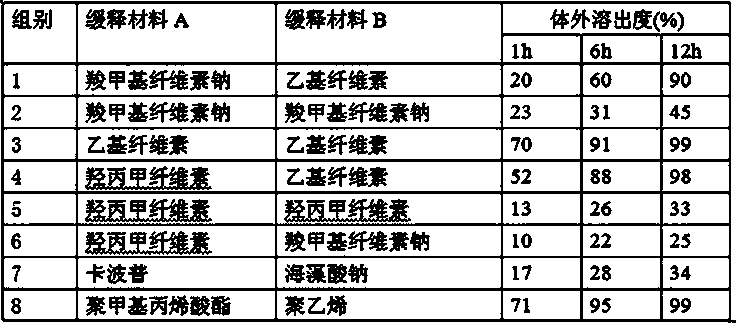
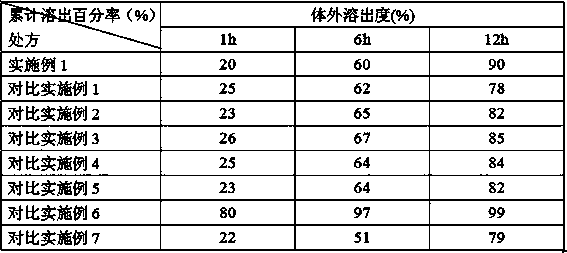
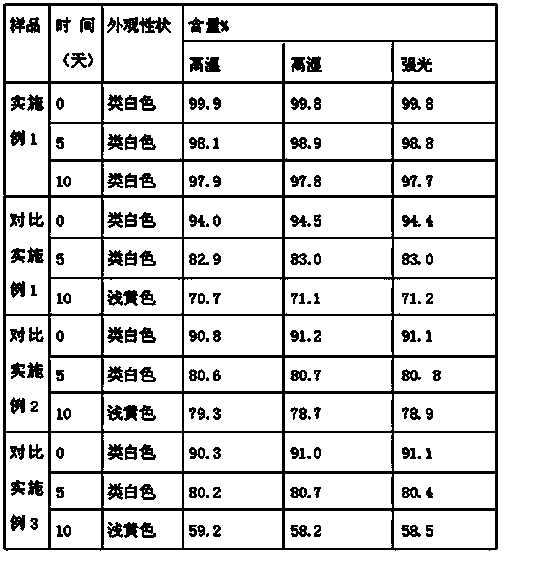
[0036] Example 1 Prescription
[0037] Tamoxifen citrate inclusion compound: Tamoxifen citrate: α-cyclodextrin ratio is 1:2
[0038] Tamoxifen citrate inclusion compound 24.5 parts
[0039] The composition of mannitol and microcrystalline cellulose (the weight ratio of the two is 6:2.3) 27 parts
[0040] 4 parts of 3% polyvinylpyrrolidone 95% ethanol solution
[0041] 11 parts methylcellulose
[0042] Composition of crospovidone and sodium carboxymethyl starch (the weight ratio of the two is 4:7.5) 14 parts
[0044] Sodium carboxymethyl cellulose 17 parts
[0045] 12 parts ethyl cellulose
[0046] Preparation:
[0047] 1. Prepare tamoxifen citrate inclusion compound according to the following method:
[0048] (1) In water or aqueous ethanol medium, react tamoxifen citrate with α-cyclodextrin in a certain proportion, filter the resulting solution through a microporous membrane until clarified, and separate the inclusion complex from the mixt...
Embodiment 2
[0053] Fully grind tamoxifen citrate and α-cyclodextrin described in Example 1 in 50% ethanol to form a uniform paste, and obtain a white powder after vacuum drying. Tamoxifen citrate increased 12-fold.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com