Tin-manganese-titanium catalyst, and preparation method and application method thereof
An application method and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., to achieve the effects of low energy consumption, high resistance to chlorine poisoning, and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
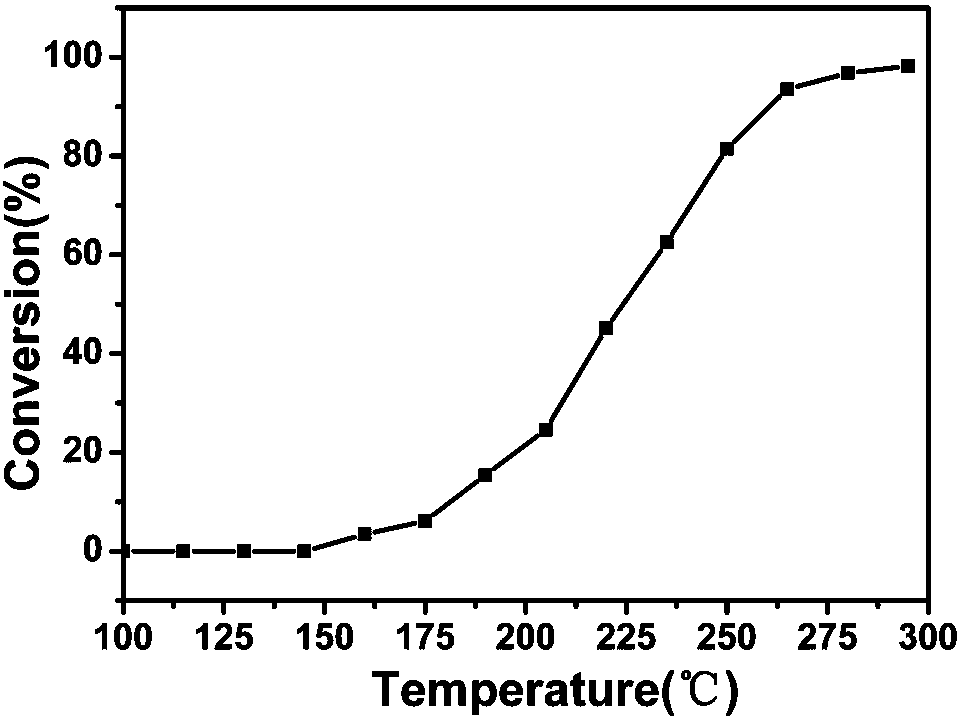
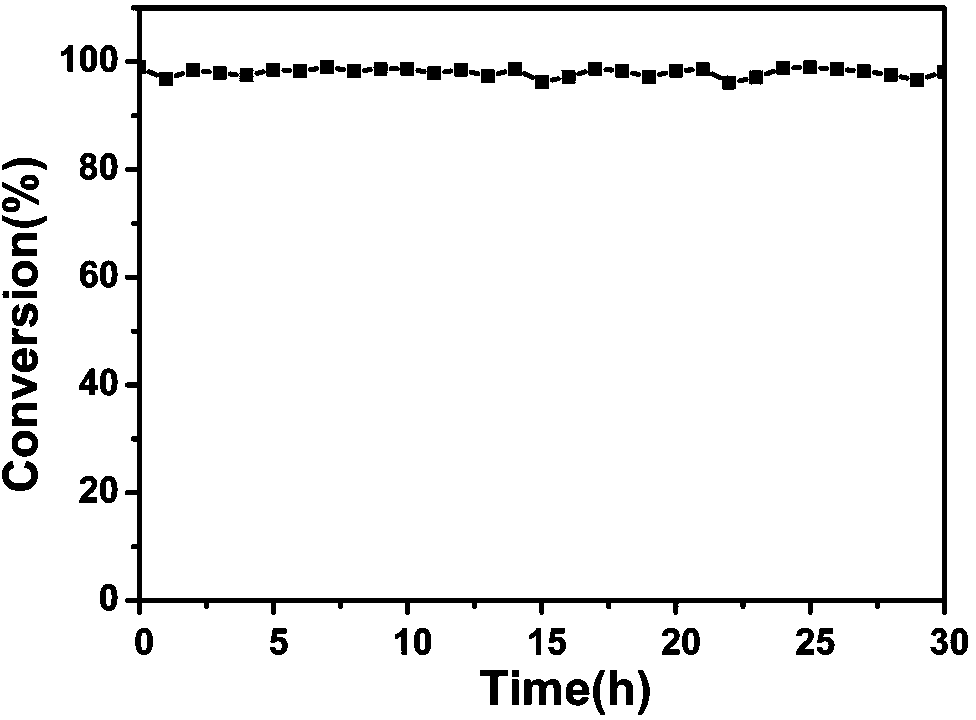
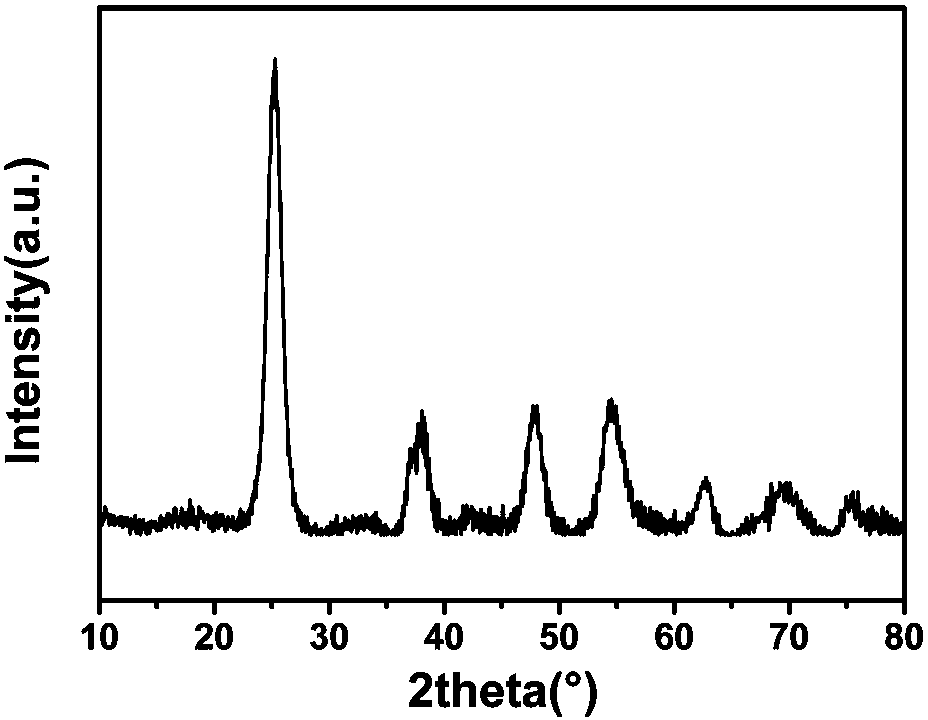
Image
Examples
Embodiment 1
[0045] The preparation method of tin-manganese-titanium catalyst comprises the following steps:
[0046] 1) Weigh Mn(NO 3 ) 2 and Ti(SO 4 ) 2 , according to Mn(NO 3 ) 2 and Ti(SO 4 ) 2 The sum of the amount of substance and SnCl 2 2H 2 The ratio of the amount of O to substance is 1.00:0.01 to weigh SnCl 2 2H 2 O;
[0047] The precursor SnCl of each component of the catalyst that weighed 2 2H 2 O, Mn(NO 3 ) 2 and Ti(SO 4 ) 2 Dissolve in an appropriate amount of ultrapure water (the quality of ultrapure water in this embodiment is the precursor SnCl 2 2H 2 O, Mn(NO 3 ) 2 and Ti(SO 4 ) 2 10 times the total mass), then add precipitant urea and dispersant polyethylene glycol to the aqueous solution of the precursor, the amount of urea added is to precipitate all Ti 4+ , Mn 2+ , Sn 2+ 5 times the amount of urea required for ions; the mass of polyethylene glycol is the precursor SnCl 2 2H 2 O, Mn(NO 3 ) 2 and Ti(SO 4 ) 2 0.5 times the total mass; put th...
Embodiment 2
[0054] The preparation method of tin-manganese-titanium catalyst comprises the following steps:
[0055] 1) Weigh Mn(NO 3 ) 2 and Ti(SO 4 ) 2 , according to Mn(NO 3 ) 2 and Ti(SO 4 ) 2 The sum of the amount of substance and SnCl 2 2H 2 The ratio of the amount of O to substance is 1:2 to weigh SnCl 2 2H 2 O;
[0056] The precursor SnCl of each component of the catalyst that weighed 2 2H 2 O, Mn(NO 3 ) 2 and Ti(SO 4 ) 2 Dissolve in an appropriate amount of ultrapure water, then add precipitant urea and dispersant polyethylene glycol to the aqueous solution of the precursor, the amount of urea added is to precipitate all Ti 4+ , Mn 2+ , Sn 2+ 25 times the amount of urea required for ions; the mass of polyethylene glycol is the precursor SnCl 2 2H 2 O, Mn(NO 3 ) 2 and Ti(SO 4 ) 2 0.2 times the total mass; put the above-mentioned prepared solution in a water bath, fully stir at 70°C for 5 h, the pH of the solution rises slowly, and a brown precipitate and m...
Embodiment 3
[0063] The preparation method of tin-manganese-titanium catalyst comprises the following steps:
[0064] 1) Weigh Mn(NO 3 ) 2 and Ti(SO 4 ) 2 , according to Mn(NO 3 ) 2 and Ti(SO 4 ) 2 The sum of the amount of substance and SnCl 2 2H 2 The ratio of the amount of O to substance is 10:1 and weighed SnCl 2 2H 2 O;
[0065] The precursor Mn(NO 3 ) 2 and Ti(SO 4 ) 2 Dissolve in an appropriate amount of ultrapure water, then add precipitant urea and dispersant polyethylene glycol to the aqueous solution of the precursor, the amount of urea added is to precipitate all Ti 4+ , Mn 2+ , Sn 2+ 23 times the amount of urea required by the ion; polyethylene glycol is added in an appropriate amount according to the volume of the solution. Put the above prepared solution in a water bath, fully stir at 90°C for 10 h, the pH of the solution rises slowly, and a brown precipitate and mother liquor are obtained;
[0066] 2) Continue to age the brown precipitate in the mother liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com