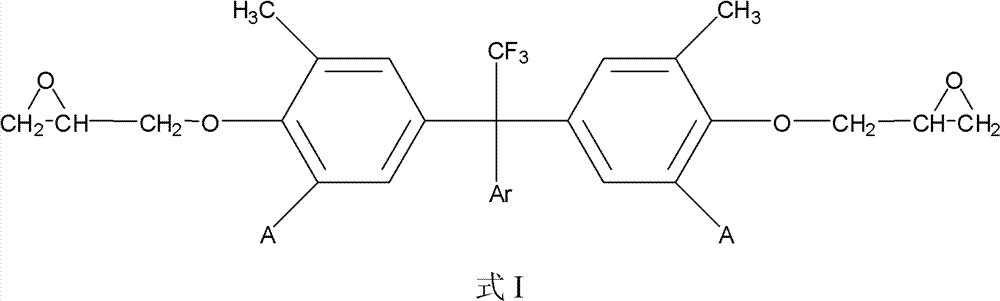
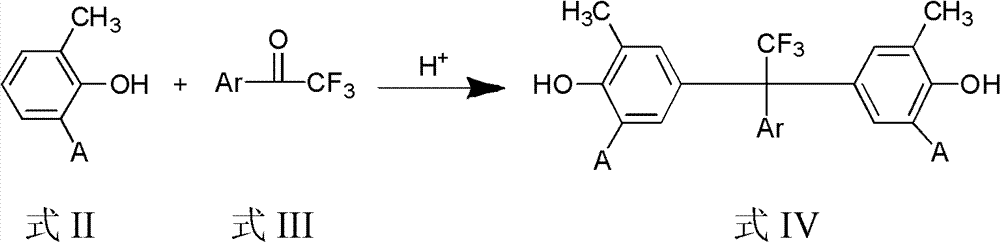
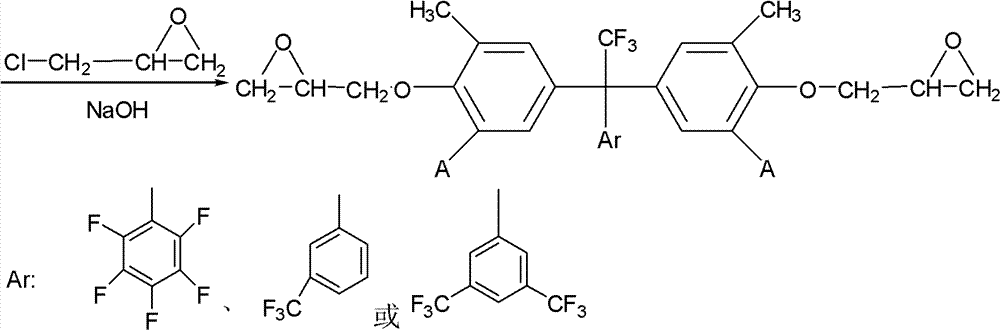
Fluorine-containing epoxy resin and preparation method thereof
An epoxy resin and glycidyl technology, applied in the direction of organic chemistry, etc., can solve the problems of easy contamination of the surface of the cured product, high surface energy of epoxy resin, limited application of epoxy resin, etc., and achieves high glass transition temperature, The effect of strong hydrophobicity and low water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The following examples are the preparation method of fluorine-containing epoxy resin and the properties of its cured product are attached to illustrate the excellent performance of the fluorine-containing epoxy resin after curing.
[0023] Characterization and test methods: Infrared spectrum is measured by Perkin-Elmer 782 Fourier transform infrared spectrometer, the sample preparation method is KBr tablet method or thin film method, and the measuring range is 4000~400cm -1 . The electrical properties were tested using a Hewlett-Packard 4284A Presion LCR tester. The dielectric constant and dielectric loss are measured by LKI-1 capacitance meter, the temperature is 25°C, and the frequency is 1MHz. Dynamic mechanical analysis (DMA) test adopts the Q-800 type instrument measurement of American TA company, glass transition temperature (T g ) is represented by the temperature corresponding to the peak of the loss tangent (tanδ).
Embodiment 1
[0025] 1,1-bis[3-methyl-4-(2,3-epoxypropyl)phenyl]-1-(2',3',4',5',6'-pentafluorophenyl) -2,2,2-Trifluoroethane
[0026] At room temperature, 132 grams of 2-methylphenol, 10 grams of trifluoromethanesulfonic acid and 66 grams of 2,3,4,5,6-pentafluoro-α, α, α-trifluoroacetophenone in a 250ml reaction bottle Mix evenly, heat to 50°C and stir for about 1 hour to completely dissolve 2-methylphenol to form a homogeneous liquid. Raise the temperature to 120°C and stir, carry out the condensation reaction under the catalysis of trifluoroacetic acid for 6 to 8 hours, and obtain a brownish-red liquid, which is distilled under reduced pressure to remove excess 2-methylphenol, and the crude product is dissolved in ethanol and water (ethanol: the volume of water Recrystallization was carried out in a mixed solution with a ratio of 1:1), and 98.175 g of white solid was obtained, with a yield of 85%.
[0027] 100 grams of the above white solid and 400 grams of epichlorohydrin were stirred ...
Embodiment 2
[0031] 1,1-bis[3,5-dimethyl-4-(2,3-epoxypropyl)phenyl]-1-(2',3',4',5',6'-pentafluoro Phenyl)-2,2,2-trifluoroethane
[0032]At room temperature, 90 grams of 2,6-dimethylphenol, 5 grams of trifluoromethanesulfonic acid and 48 grams of 2,3,4,5,6-pentafluoro-α, α, α-trifluoroacetophenone in 250ml After mixing evenly in the reaction flask, heat to 50°C and stir for about 1 hour to completely dissolve 2,6-dimethylphenol to form a homogeneous liquid. Warm up to 120°C and stir, and condense under the catalysis of trifluoromethanesulfonic acid for 12 hours to obtain a brownish-red liquid, which is distilled off under reduced pressure to remove excess 2,6-xylenol, and the crude product is dissolved in ethanol and water (ethanol: water Recrystallization was carried out in the mixed solution whose volume ratio was 4:1), and 71 g of white solid was obtained, and the yield was 80%.
[0033] Stir and mix 100 grams of the above-mentioned white solid with 800 grams of epichlorohydrin, add 65...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Volume resistivity | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com