Multi-parameter immunochromatographic assay test paper and preparation method thereof
An immunochromatographic detection, multi-parameter technology, applied in measurement devices, analytical materials, instruments, etc., can solve the problems of lower sensitivity than single detection, increased colloidal gold dosage, and low sensitivity, etc., to shorten detection time, improve efficiency, The effect of saving manpower and material resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example describes the preparation of solid-phase membranes and conjugate pad membranes by printing:
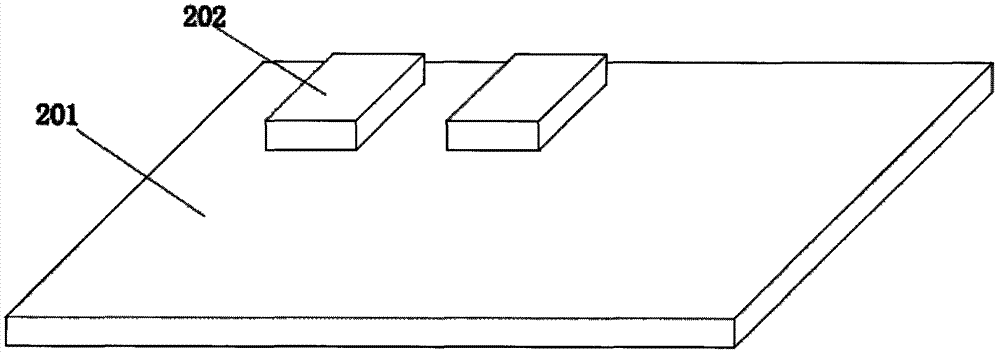
[0041] Dissolve the solid-phase membrane material in a solvent, add additives, including surfactants, high molecular polymers, salt ions, and forming agents, and dissolve them in a buffer system to form ink. The ink concentration range is 10%-70% solid content, the viscosity is 0.1-15Pa.s, and it will not flow away from the substrate during the film-forming process.
[0042] The film material of the bonding pad is dissolved in a solvent, and additives are added, including microcrystalline cellulose, microcrystalline cellulose glue or sodium carboxymethyl cellulose, and hydrogenated lecithin to form an ink. The ink concentration range is 10%-70% solid content, the viscosity is 0.1-15Pa.s, and it will not flow away from the substrate during the film-forming process.
[0043] Use an automatic or semi-automatic printing machine to print the above ink on the surface of...
Embodiment 2
[0050] This example describes the preparation of solid-phase membranes and conjugate pad membranes by separating continuous thin films
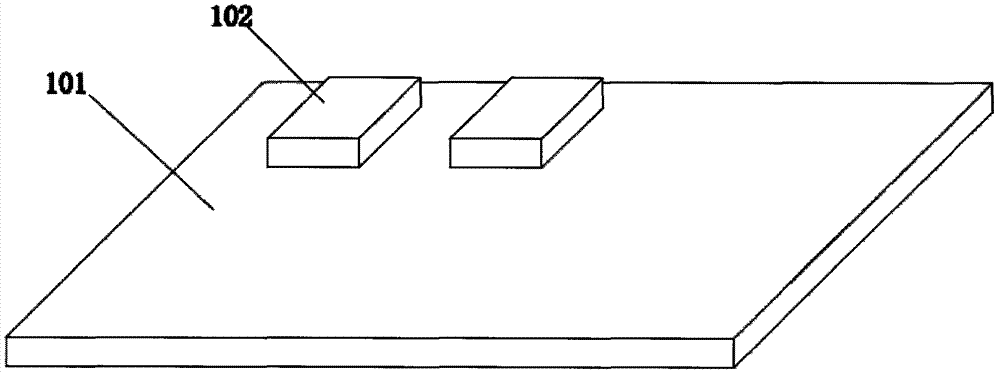
[0051] The solid-phase film can adopt the finished product of commercial solid-phase film coated with a large area, and print ink on the surface of the film by printing methods, such as UV ink, volatilization curing ink, heat curing ink; and / or adopt die-cutting technology, including mechanical die-cutting , laser cutting technology, ultrasonic stripping technology, destroy the membrane structure or / and membrane material at the corresponding position, make it lose hydrophilicity and chromatography performance, and separate the continuous film into fluid-interconnected materials. The above processing process can be selected before or after the solid-phase membrane is fixed on the immunochromatographic detection test paper liner coated with adhesive.
[0052] Before or after the solid-phase membrane is fixed on the immunochromatography test pap...
Embodiment 3
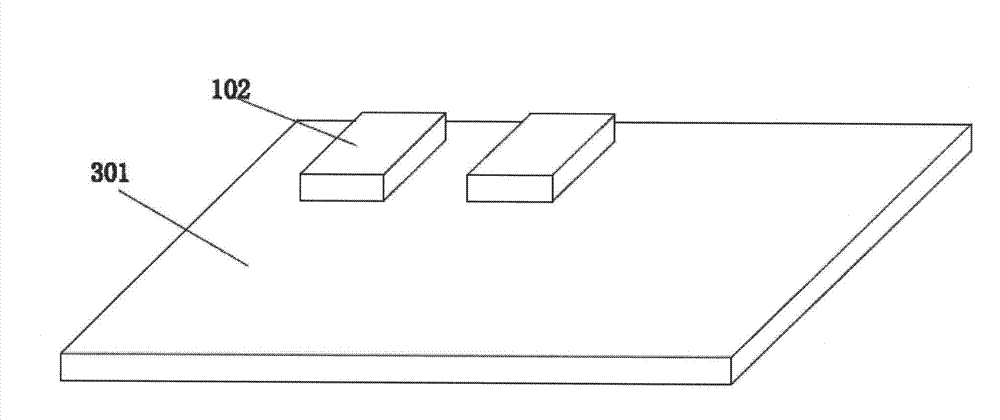
[0057] This embodiment is to describe the cross-combination use of printing method film making and separated continuous film method film making:
[0058] Preparation of solid-phase membranes by printing and separation of continuous thin films to prepare conjugation pad membranes;
[0059] Separated continuous thin film method to prepare solid phase membrane + printing method to prepare conjugation pad membrane.
[0060] Absorbent cushion film and sample cushion film can directly use commercially available products. The sample cushion membrane is mostly made of glass fiber or cellulose filter membrane, and some whole blood separation materials are used as the sample cushion membrane. Referring to the preparation method of the conventional colloidal gold immunochromatography test paper, the water-absorbing pad film and the sample pad film are cut into long strips and pasted on the appropriate position of the immunochromatography test paper liner on which the solid-phase film an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com