1-amino-3-alkyl-1, 2, 3-triazole nitrate and its synthesis method
A synthetic method and nitrate technology, applied in organic chemistry, explosives, etc., to achieve low cost and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Under solvent-free conditions, add 0.15 mol of hydrazine hydrate into the reactor, slowly add 0.05 mol of glyoxal (40% aqueous solution) dropwise into the reactor, react at 75°C for 4 hours, and dissolve the reaction solution Distilled under reduced pressure, and the solid was washed with isopropanol to obtain glyoxal dihydrazone;
[0029] (2) Add 30mL of solvent water, add 5g of glyoxal dihydrazone into the reactor, slowly add 50mL of hydrogen peroxide with 30% catalyst, react at 15°C for 3h, and distill the reaction solution under reduced pressure to obtain 1-amino- 1,2,3,-triazole;
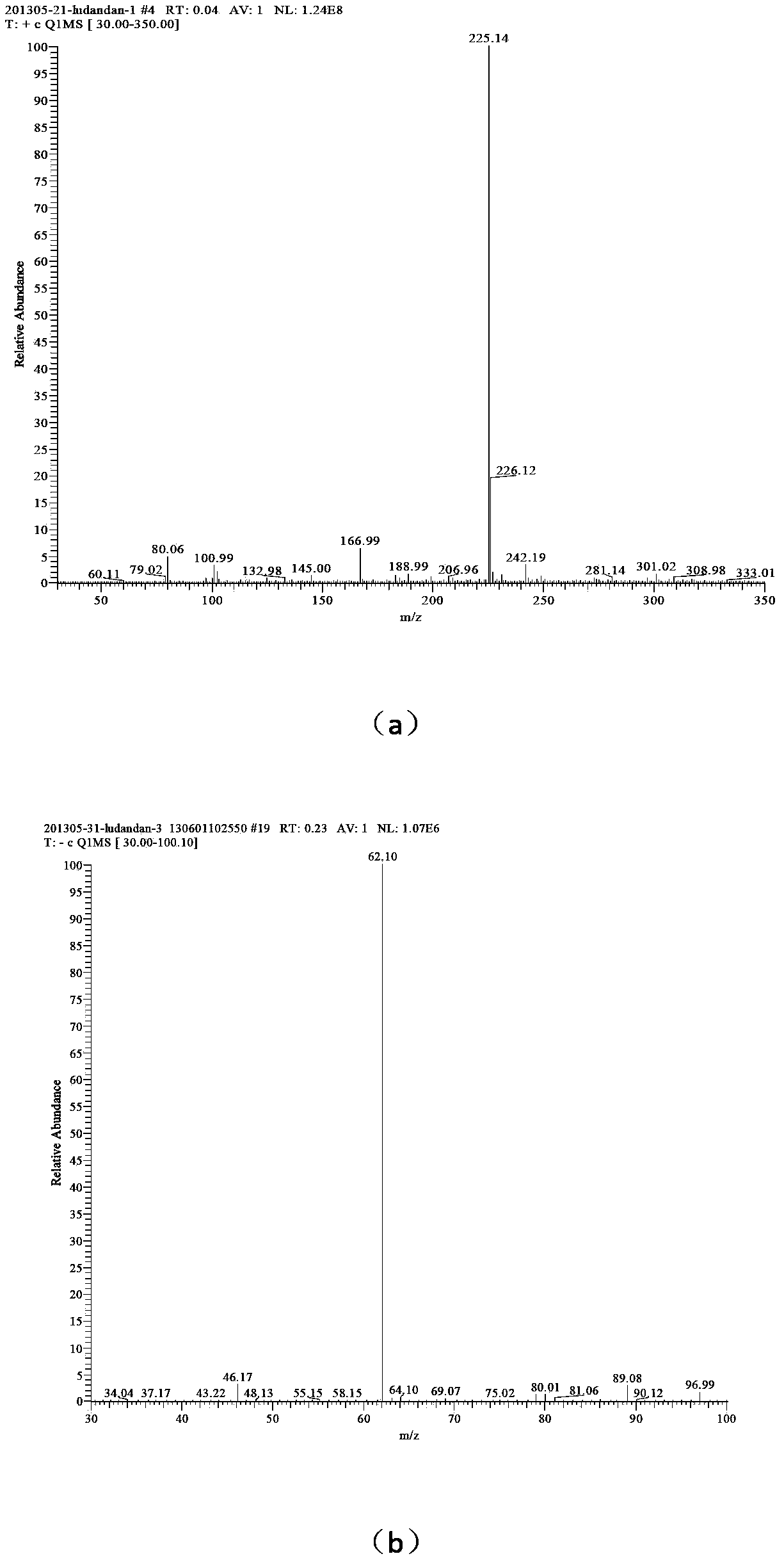
[0030] (3) Add 25 mL of solvent water, add 0.01 mol of 1-amino-1,2,3,-triazole into the reactor, add 0.01 mol of n-decane bromide, add 0.0066 g of phase transfer catalyst tetrabutylammonium chloride, and React at 60°C for 30 hours, distill the reaction solution under reduced pressure, wash the solid three times with ethyl acetate to obtain 1-amino-3-n-decyl-1,2,3-triazole bromide; ...
Embodiment 2
[0033] (1) and (2) are exactly the same as in Example 1.
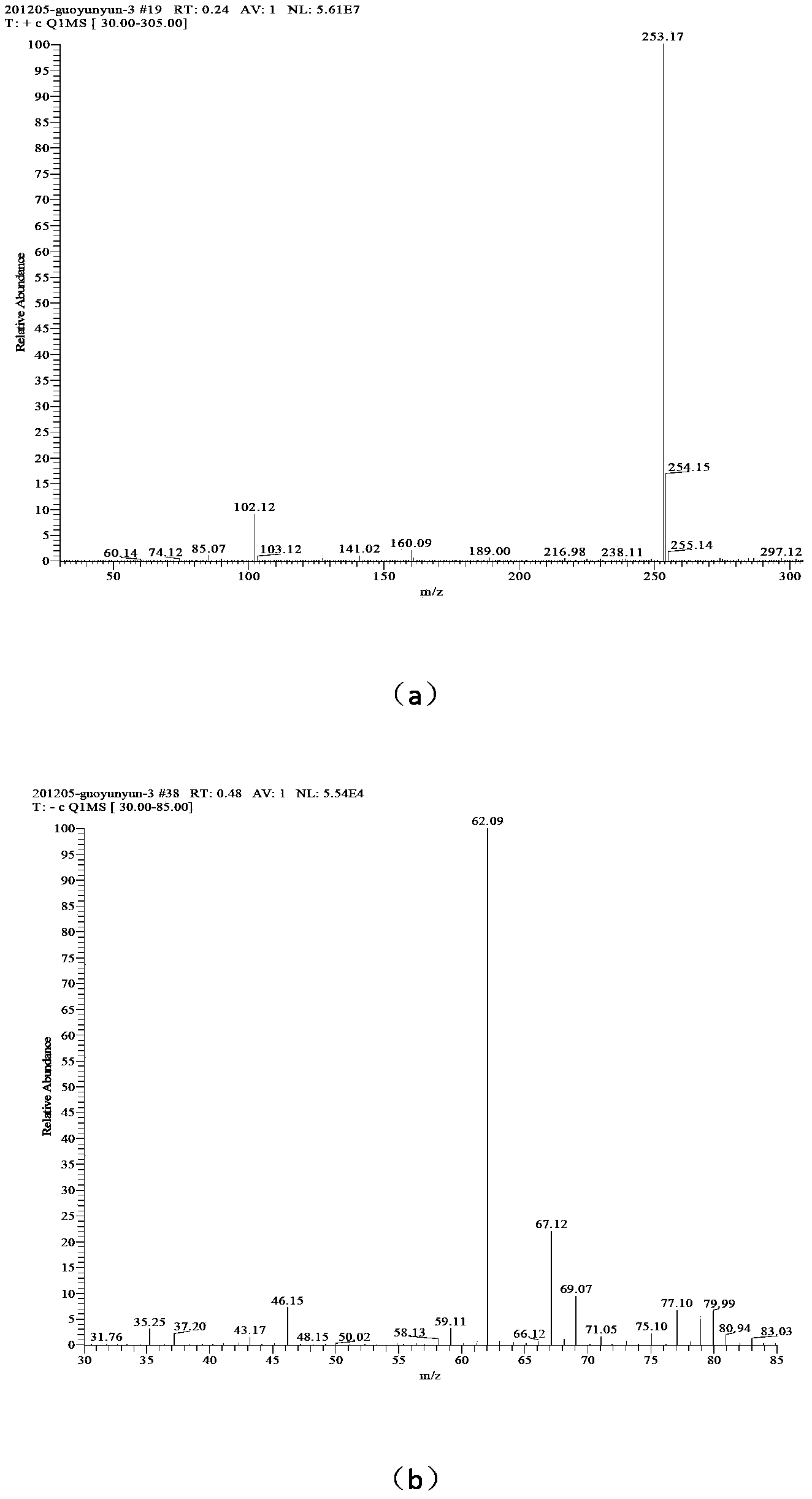
[0034] (3) Add 42mL of solvent water, add 0.01mol of 1-amino-1,2,3,-triazole into the reactor, add 0.02mol of n-bromodecane, and add a small amount of phase transfer catalyst tetrabutylammonium bisulfate 0.2211g , reacted at 80°C for 40 h, distilled the reaction solution under reduced pressure, washed the solid three times with ethyl acetate to obtain 1-amino-3-n-decyl-1,2,3-triazole bromide;
[0035] (4) Add 46 mL of solvent water, add 0.01 mol of 1-amino-3-n-decyl-1,2,3-triazole bromide into the reactor, dissolve 0.01 mol of silver nitrate in 10 mL of water, and slowly drop Add it to the container, react for 40 minutes, filter to remove silver bromide, and distill the reaction solution under reduced pressure to obtain 1-amino-3-n-decyl-1,2,3-triazole nitrate, the confirmation of the compound of this example The method is the same as in Example 1.
Embodiment 3
[0037] (1) and (2) are exactly the same as in Example 1.
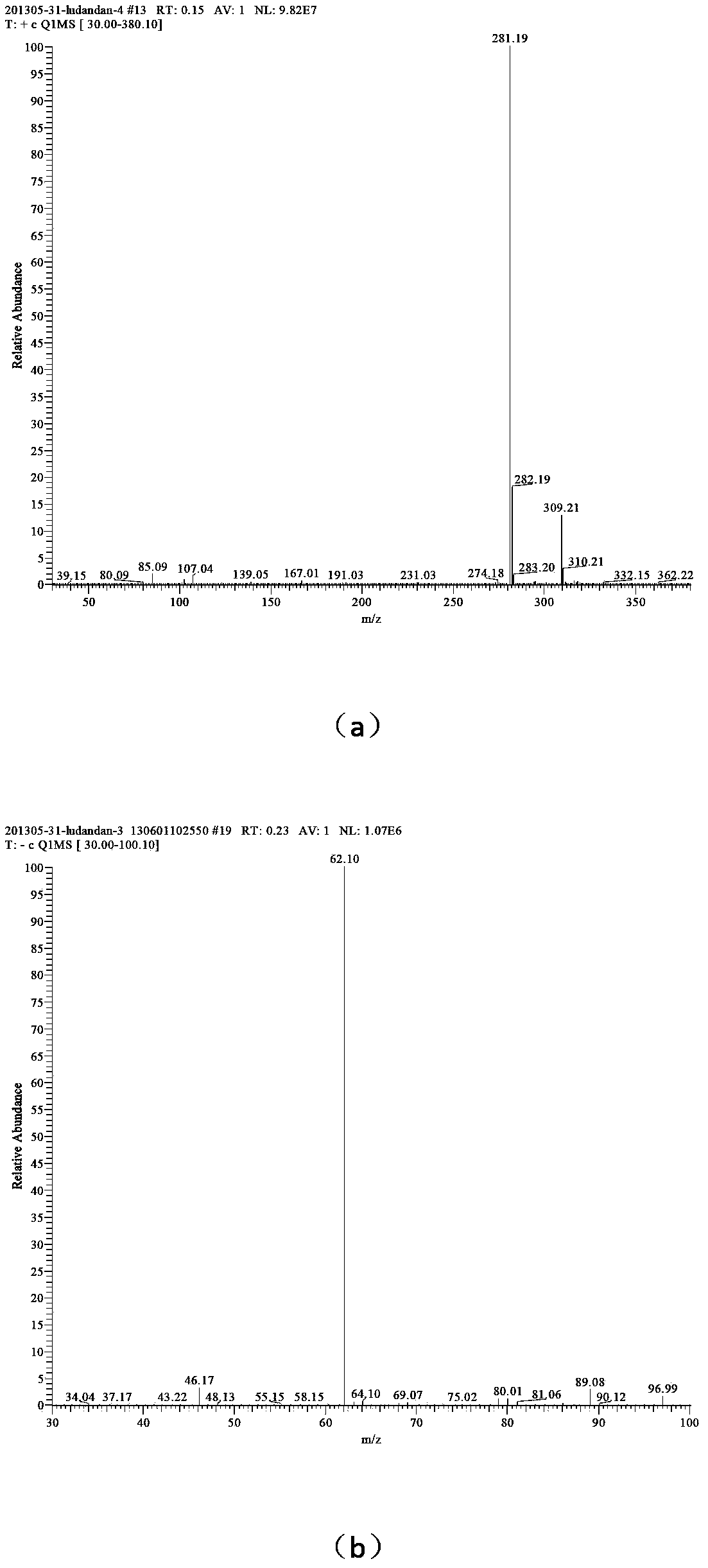
[0038] (3) Add 25 mL of solvent water, add 0.01 mol of 1-amino-1,2,3,-triazole into the reactor, add 0.01 mol of dodecane bromide, add 0.0075 g of phase transfer catalyst tetrabutylammonium chloride, and React at 60°C for 30 hours, distill the reaction solution under reduced pressure, wash the solid with ethyl acetate three times to obtain 1-amino-3-dodecyl-1,2,3-triazole bromide;
[0039](4) Add 33 mL of solvent water, add 0.01 mol of 1-amino-3-dodecyl-1,2,3-triazole bromide into the reactor, dissolve 0.01 mol of silver nitrate in 10 mL of water, and slowly drop Add it to the container, react for 20min, remove silver bromide by filtration, and distill the reaction solution under reduced pressure to obtain 1-amino-3-dodecyl-1,2,3-triazole nitrate, whose mass spectrum is as follows figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com