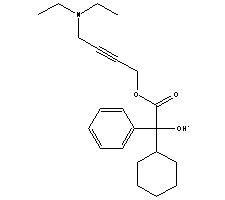
Oxybutynin chloride externally-applied preparation and preparation method thereof
An external preparation, oxybutynin technology, applied in the field of pharmaceutical preparations, can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of oxybutynin granule intermediate adopts following prescription:
[0036] Oxybutynin 5g
[0037] Medium Chain Fatty Acid Ester 95g
[0038] Lecithin 20g
[0039] Tween 80 10g
[0040] water 370g
[0041] Prepared fat milk 500g
[0042] Hypromellose 200g
[0043] Sucrose 50g
[0044] Prepared into granules 380g
[0045] Preparation Process:
[0046] 1. Raw and auxiliary materials are pretreated, oxybutynin is crushed to 300 mesh, and other solid auxiliary materials are crushed to 200 mesh;
[0047] 2. Disperse oxybutynin in the medium-chain fatty acid ester, heat and melt, keep the temperature at 70°C, and form the oil phase;
[0048] 3. Weigh lecithin and Tween-80 in water at 75°C, stir and disperse evenly, forming the water phase;
[0049] 4. Slowly add the oil phase into the water phase under low-speed stirring, emulsify until there are no oil droplets on the surface, and then get colostrum;
[0050] 5. High-speed shearing of...
Embodiment 2
[0055] Embodiment 2: the preparation of oxybutynin granule intermediate adopts following prescription:
[0056] Oxybutynin 5g
[0057] soybean oil 55g
[0058] Span 80 10g
[0059] Tween 80 10g
[0060] water 220g
[0061] Made into fat milk 300g
[0062] Sucrose 60g
[0063] Mannitol 60g
[0064] Makes 110g
[0065] Preparation Process:
[0066] 1. Raw and auxiliary materials are pretreated, oxybutynin is crushed to 300 mesh, and other solid auxiliary materials are crushed to 200 mesh;
[0067] 2. Disperse oxybutynin in soybean oil and Span 80, heat and melt, keep the temperature at 80°C, and form the oil phase;
[0068] 3. Weigh Tween-80 and dissolve it in water at 85°C to form the water phase;
[0069] 4. Slowly add the oil phase into the water phase under low-speed stirring, emulsify until there are no oil droplets on the surface, and then get colostrum;
[0070] 5. High-speed shearing of colostrum in a high-pressure homogenizer to obtain fat emulsion;
[...
Embodiment 3
[0075] Embodiment 3: Preparation of dry milk type oxybutynin granules
[0076] The oxybutynin granule intermediate 380g of embodiment 1 gained
[0077] Povidone 180g
[0078] Sodium carboxymethyl starch 395g
[0079] Fragrance 20g
[0080] Make 1000 bags
[0081] Preparation Process:
[0082] 1. Pretreatment of auxiliary materials, passing through a 200-mesh sieve;
[0083] 2. The oxybutynin granule intermediate obtained in Example 1 is mixed homogeneously with other auxiliary materials;
[0084] 3. Test and package.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com