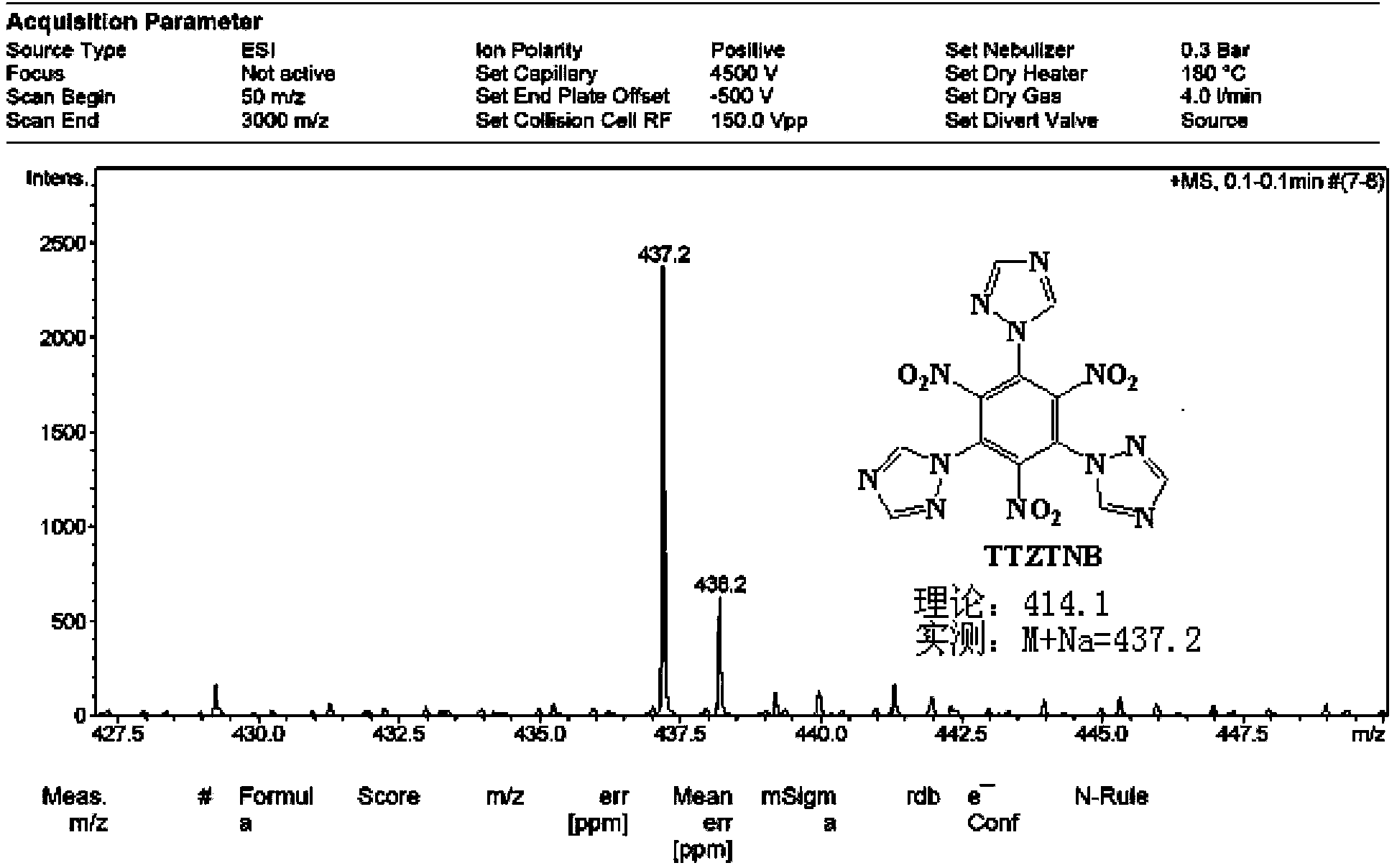
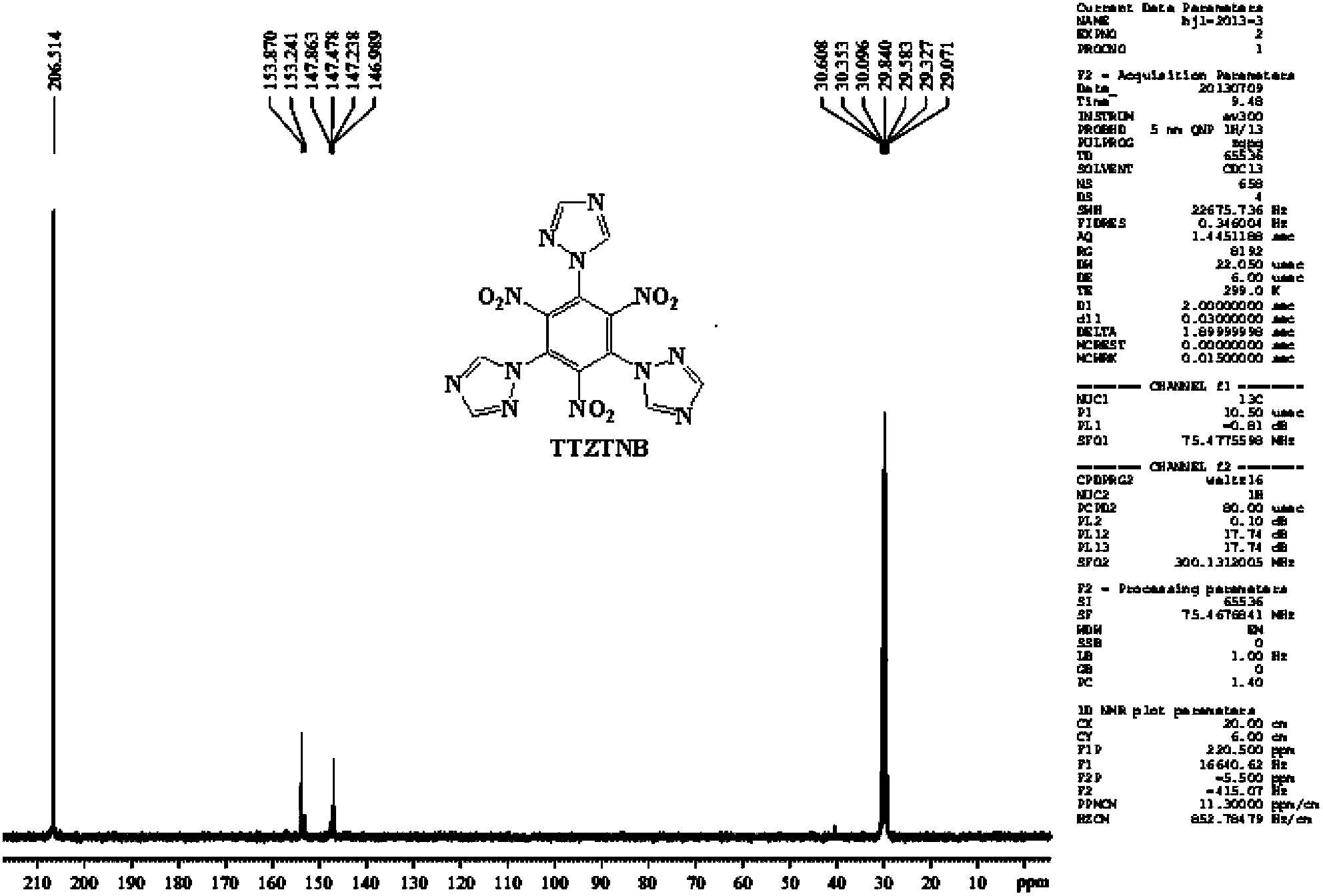
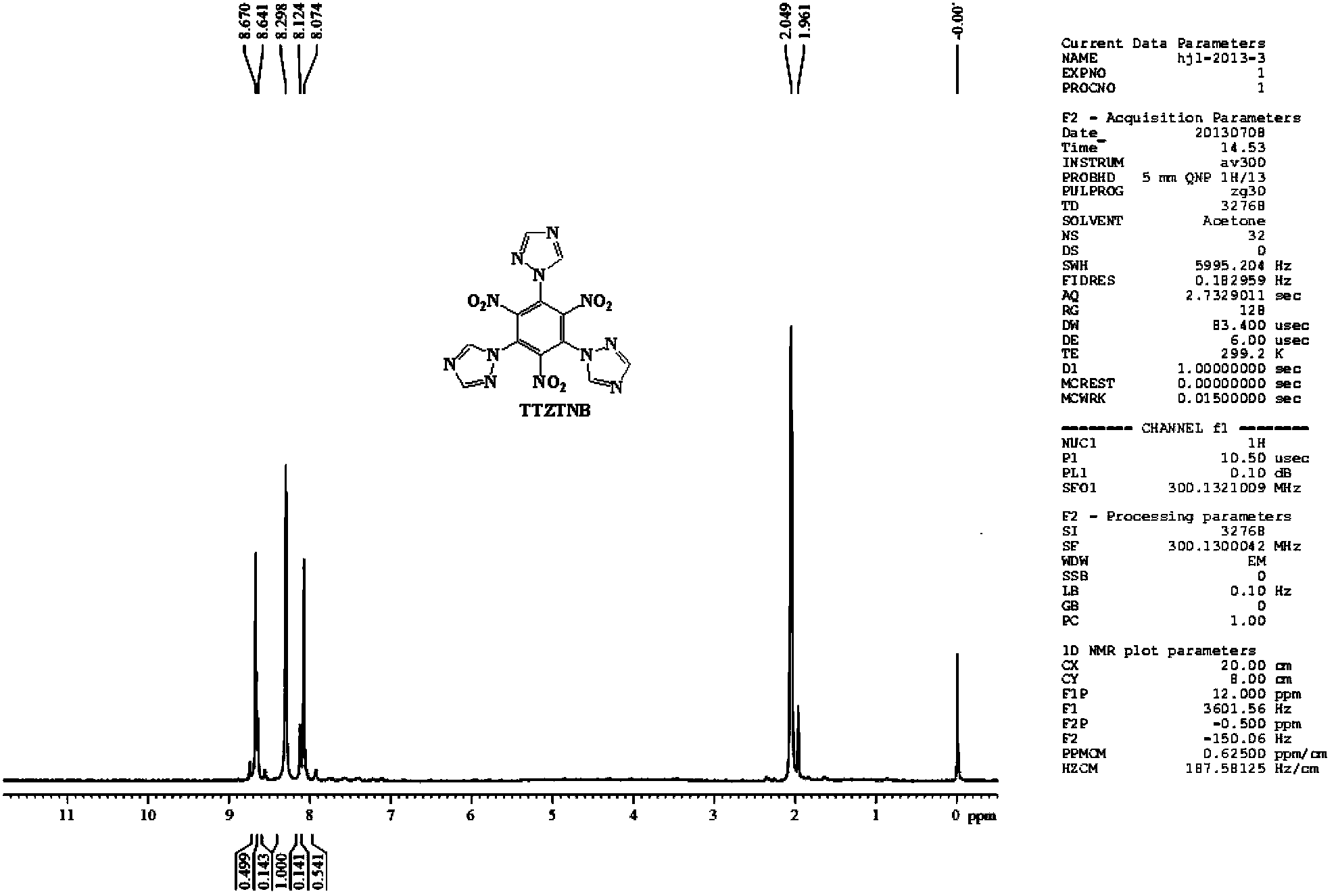
Energetic compound 1,3,5-three(1H-1,2,4-triazolyl)-2,4,6-trinitrobenzene and synthetic method thereof
A technology of trinitrobenzene and a synthesis method, which is applied in the direction of organic chemistry and the like, can solve the problems of loose molecular stack structure and low nitrogen content, and achieves the effects of high energy density, simple reaction steps and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] At room temperature, add 15ml of dimethylformamide (DMF) solvent into a 50mL three-necked reaction flask equipped with a drying tube, turn on the magnetic stirrer, and weigh 0.949g (3mmol) of 1,3,5-trichloro-2 , Add 4,6-trinitrobenzene into the reaction flask, then weigh 0.683g (9mmoL) of 1H-1,2,4-triazole into the reaction flask, and raise the temperature to 45-50°C. After all the solids are dissolved, weigh 1.368g (9.9mmoL) of potassium carbonate (K 2 CO 3 ) as a catalyst and slowly added to the reaction flask several times, the addition time is 20-25min, and the reaction is kept at this temperature. After thin-layer chromatography detects that there is no raw material point, the solid-liquid mixture is filtered under reduced pressure, and solids such as potassium carbonate are filtered off, washed twice with 10 mL of DMF solvent, and the filtrate is evaporated with a rotary evaporator to remove the DMF solvent, and then filtered with a chromatographic column Method...
Embodiment 2
[0028] At room temperature, add 15 mL of dimethyl sulfoxide (DMSO) solvent into a 50 mL three-neck reaction flask equipped with a drying tube, turn on the magnetic stirrer, and weigh 0.949 g (3 mmoL) of 1,3,5-trichloro-2 , Add 4,6-trinitrobenzene into the reaction flask, then weigh 0.683g (9mmoL) of 1H-1,2,4-triazole into the reaction flask, and raise the temperature to 45-50°C. After all the solids are dissolved, weigh 1.368g (9.9mmoL) of potassium carbonate (K 2 CO 3 ) as a catalyst and slowly added to the reaction flask several times, the addition time is 20-25min, and the reaction is kept at this temperature. When the thin-layer chromatography detects that there is no raw material point, the solid-liquid mixture is filtered under reduced pressure to remove solids such as potassium carbonate, and is washed twice with 10 mL of DMSO solvent. Methods for separation, the developer used a mixed solvent of petroleum ether and ethyl acetate, the volume ratio of petroleum ether: ...
Embodiment 3
[0030] At room temperature, add 15mL of acetone solvent into a 50mL three-neck reaction flask equipped with a drying tube, turn on the magnetic stirrer, and weigh 0.949g (3mmoL) of 1,3,5-trichloro-2,4,6-trinitrate Add phenylbenzene into the reaction flask, then weigh 0.683g (9mmoL) of 1H-1,2,4-triazole into the reaction flask, and raise the temperature to 45-50°C. After all the solids are dissolved, weigh 1.368g (9.9mmoL) of potassium carbonate (K2CO3) as a catalyst and slowly add it to the reaction flask several times for 20-25 minutes, and keep it at this temperature for reaction. When the thin-layer chromatography detects that there is no raw material point, the solid-liquid mixture is filtered under reduced pressure to remove solids such as potassium carbonate, and is washed twice with 10 mL of DMSO solvent. Methods for separation, the developer used a mixed solvent of petroleum ether and ethyl acetate, the volume ratio of petroleum ether: ethyl acetate = 1:6. Evaporate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com