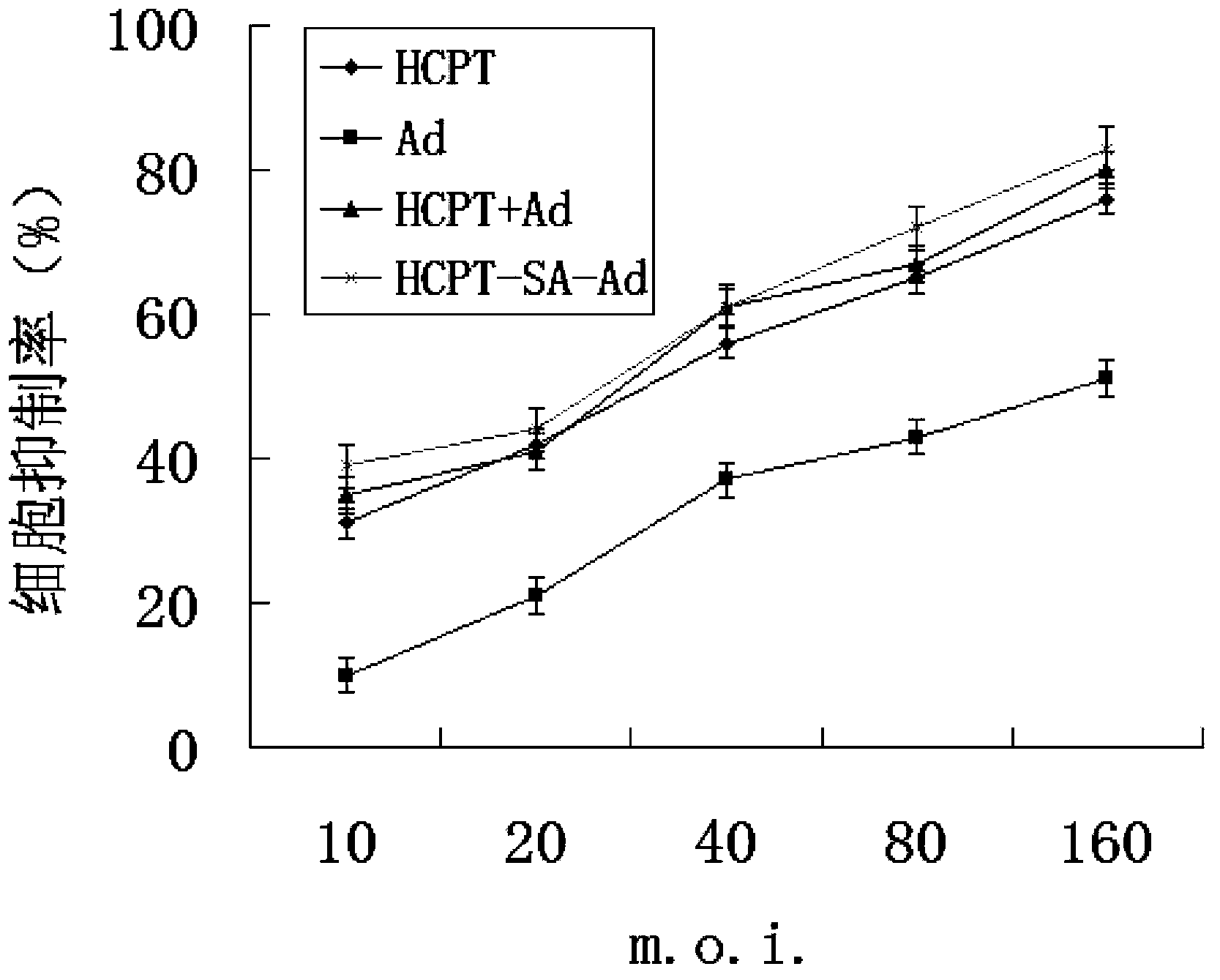
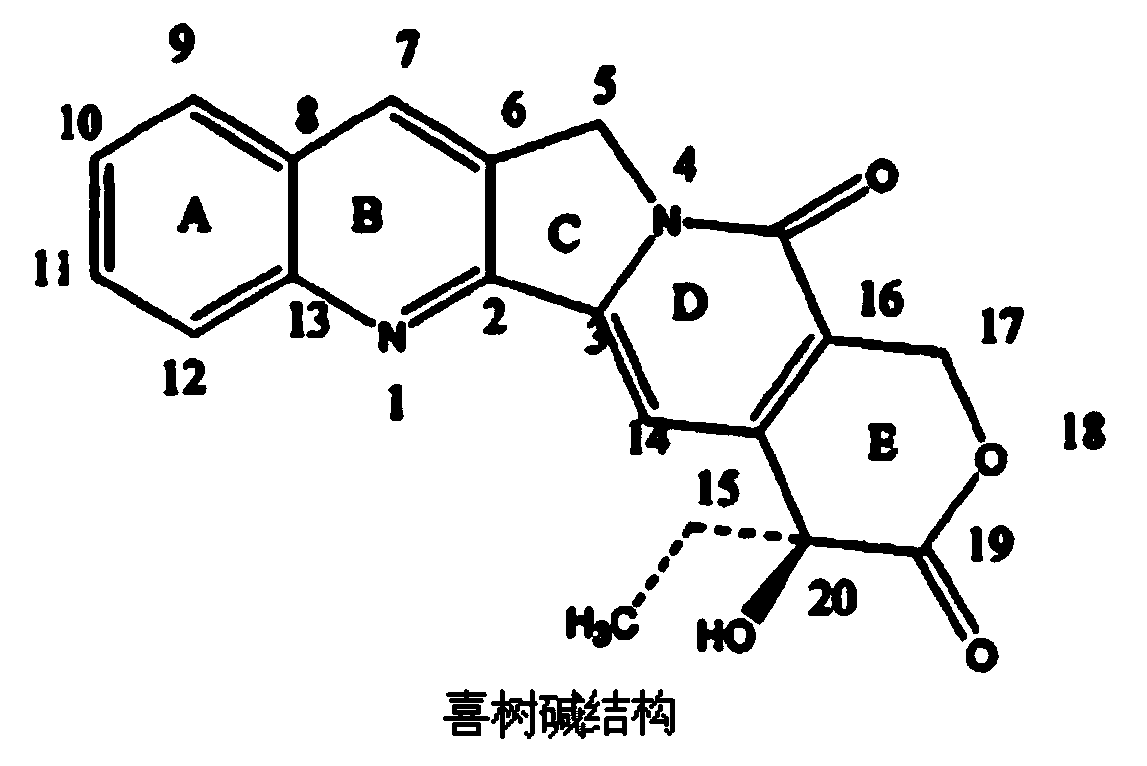
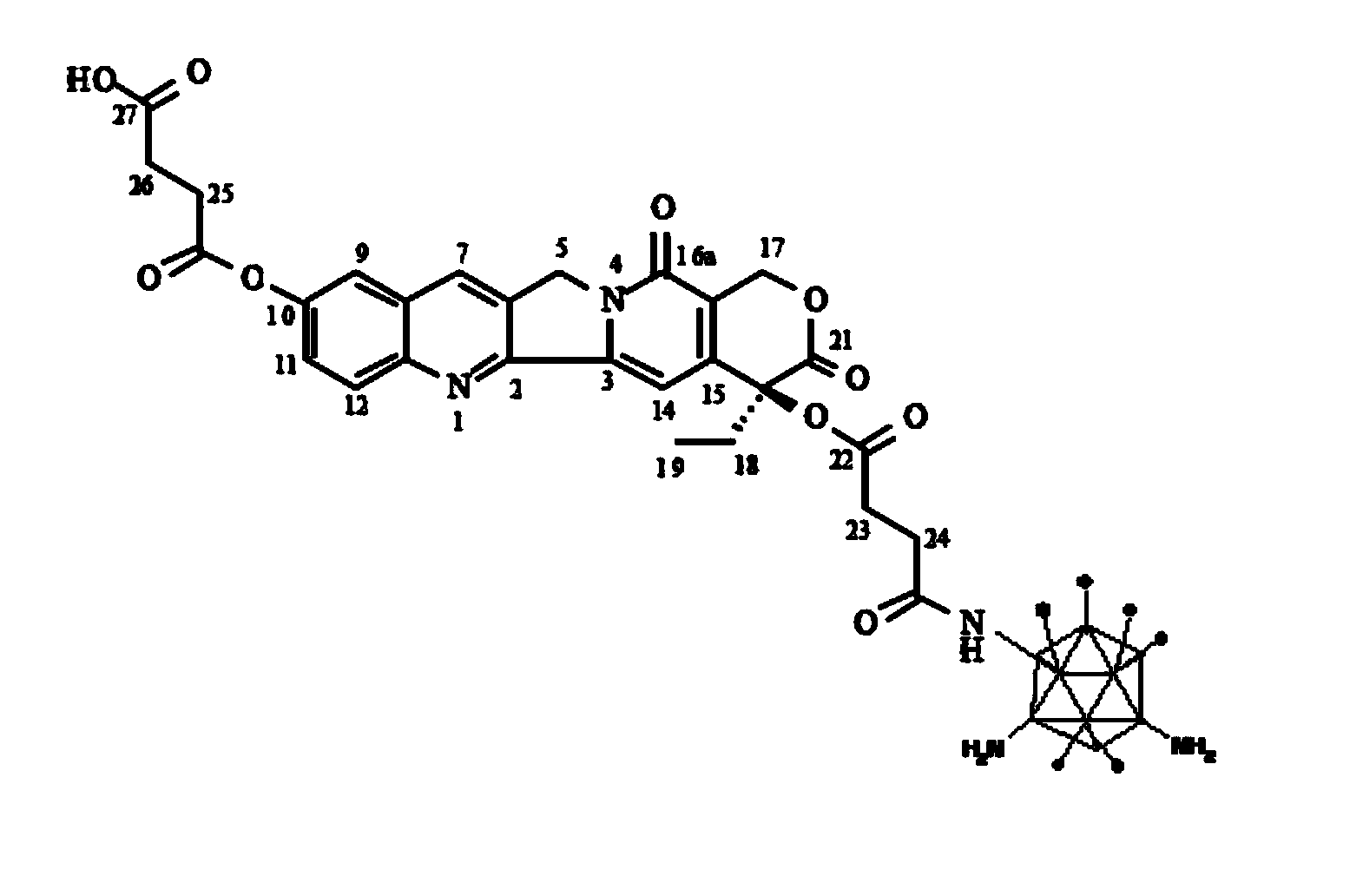
Conjugate of 10-hydroxycamptothecine-butanedioic acid and adenovirus, as well as production method and use thereof
A technology of hydroxycamptothecin and adenovirus, which is applied in the direction of medical raw materials derived from viruses/phages, drug combinations, and medical preparations containing active ingredients, etc., can solve problems such as drug resistance and affect effectiveness, and achieve reduction Dosage, improvement of killing effect, and effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of embodiment 1, 10-hydroxycamptothecin-succinic anhydride
[0043] After 10-hydroxycamptothecin (200mg, 0.247mmol) was dissolved in anhydrous pyridine (20ml), DMAP (4-dimethylaminopyridine, 123.6mg, 0.594mmol) and succinic anhydride (1180mg, 2.47mmol) were added, 60 Stir at ℃ for 72h. After the reaction product was cooled to room temperature, it was diluted with 20ml of toluene, then evaporated to dryness in a rotary vacuum (condition: 0.1Mpa, 50°C), and repeated once (ie, repeat the dilution with toluene and evaporated to dryness in a rotary vacuum). Add another 50ml of chloroform to the spinner bottle, and wash the organic phase with 20ml of 2% (mass concentration) dilute hydrochloric acid and 20ml of saturated NaCl solution, and repeat once (that is, repeatedly wash the organic phase with 20ml of 2% dilute hydrochloric acid and 20ml of saturated NaCl solution); The phase was dried with anhydrous MgSO4, filtered with suction, and concentrated to consta...
Embodiment 2
[0054] Example 2 HCPT-SA-Ad trail preparation of
[0055] HCPT-SA (15 mg, 0.027 mmol) was dissolved in DMF (N,N-dimethylformamide, 500 μL), and NHS (N-hydroxysuccinimide, 4.6 mg, 0.05 mmol) and EDAC (1-(3 -Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 4.8mg, 0.027mmol) was reacted at room temperature (10~25°C) for 4 hours. Add the reacted solution to about 2mL containing 2×10 10 Ad of pfu trail PBS (10mM, pH 7.4) solution (the molar ratio is HCPT-SA: Ad trail = 10 9 : 1 pmol), at 4 ° C, stirred dark reaction for 12 hours. After the reaction, centrifuge (5000rpm, 30min), take the supernatant, and purify the supernatant by Sephadex G-25. The specific content of the purification is: use Sephadex G-25 gel chromatography, the buffer solution is PBS (10 mM, pH 8.0), the flow rate is 1 ml / min, after equilibrating the chromatography column with 5 column volumes of PBS solution, put on After the sample, continue to add PBS, keep the flow rate at 1 ml / min, detect the ...
Embodiment 3
[0058] Example 3 HCPT and Ad trail Determination of the number of covalently bound molecules of capsid proteins
[0059] Make a standard absorption curve of HCPT monomer, and determine HCPT and Ad by calculating the recovery rate of unreacted HCPT trail The number of covalently bound molecules of amino groups on the capsid protein (n=4), and the number of unbound HCPT molecules were calculated according to the standard curve of the regression equation. In the above experiments, methanol was used as a blank. The amount of HCPT reaction is calculated based on its UV absorption value at 375nm. The standard curve regression equation established based on the UV absorption value of HCPT at 375 nm (methanol solution): Y (OD) = 112.02X (μg / mL) - 1.2959 (R 2 =0.9994), its linear range is 0.0018 ~ 15.18μg / mL, calculate the HCPT content in the eluate. Subtract the HCPT content in the purified column eluate from the amount of HCPT initially participating in the reaction to obtain HCPT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com