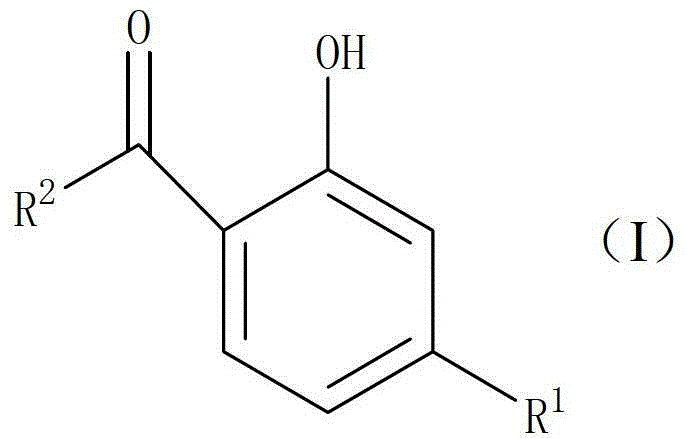
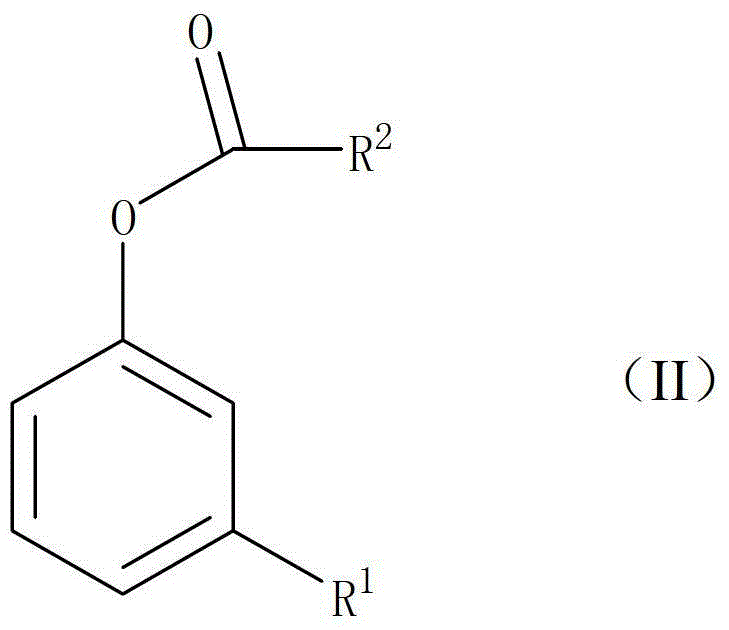
Preparation method of 2-hydroxyl-4-substituted arone compound
A technology for aryl ketones and compounds, which is applied in the field of preparation of 2-hydroxy-4-substituted aryl ketone compounds, can solve the problems of unsuitability for large-scale industrial production, difficulty in product separation and purification, and excessive production of three wastes, etc., and achieve excellent solubility performance , low process pollution and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
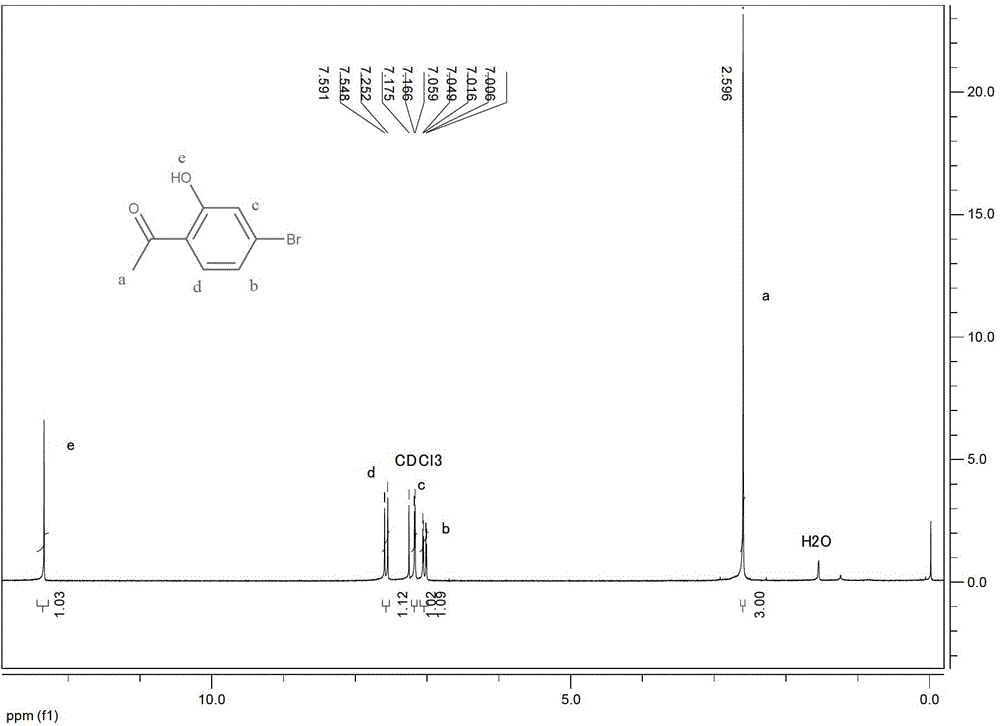
[0070] Preparation of 2-hydroxy-4-bromoacetophenone
[0071]
[0072] (1) Preparation of [sulfuric acid][butylammonium triethylamine 4-sulfonate][zinc oxide] ionic liquid
[0073] Under the condition of stirring, slowly drop 9.4g of 1,4-butane sultone into 7.0g of triethylamine, react at 80°C for 2h, cool down to 25°C, filter with suction, wash the filter cake with ethanol three times, Dry in vacuo to obtain triethylamine butane sulfonic acid inner salt.
[0074] Under the ice bath, mix the deionized aqueous solution of 15.8 g of triethylamine butane sulfonic acid inner salt obtained in the previous step and 6.5 g of sulfuric acid, react at 50 ° C for 3 h, and after cooling, vacuum dry to obtain [sulfuric acid] [triethylamine 4-butylammonium sulfonate] ionic liquid intermediate.
[0075] Under stirring conditions, 5.2 g of zinc oxide and 21.3 g of the [sulfuric acid] [triethylamine 4-butylammonium sulfonate] ionic liquid intermediate obtained in the previous step were add...
Embodiment 2
[0083] Preparation of 2-Hydroxy-4-methoxypropiophenone
[0084]
[0085] (1) Preparation of [sulfuric acid][dipropylamine 4-butylammonium sulfonate][copper oxide] ionic liquid
[0086] Under the condition of stirring, slowly drop 21.7g of 1,4-butane sultone into 16.1g of dipropylamine, react at 70°C for 2h, cool down to 25°C, filter with suction, wash the filter cake with ethanol three times, vacuum Dry to obtain dipropylamine butane sulfonic acid inner salt.
[0087] Under an ice bath, mix the deionized aqueous solution of 36.7 g of dipropylamine butane sulfonic acid inner salt obtained in the previous step with 15.2 g of sulfuric acid, react at 60° C. for 5 h, and after cooling, dry in vacuum to obtain [sulfuric acid] [dipropylamine 4- Butylammonium sulfonate] ionic liquid intermediate.
[0088] Under stirring conditions, 11.8 g of copper oxide and 49.7 g of the [sulfuric acid] [dipropylamine 4-butylammonium sulfonate] ionic liquid intermediate obtained in the previous ...
Embodiment 3
[0096] Preparation of 2-Hydroxy-4-methylphenylbenzophenone
[0097]
[0098] (1) Preparation of [sulfuric acid][dipropylamine 4-butylammonium sulfonate][copper oxide] ionic liquid
[0099] Under stirring conditions, slowly drop 14.1 g of 1,4-butane sultone into 10.4 g of dipropylamine, react at 70°C for 2 hours, cool down to 25°C, filter with suction, wash the filter cake with ethanol three times, vacuum Dry to obtain dipropylamine butane sulfonic acid inner salt.
[0100] Under ice bath, mix the deionized aqueous solution of 23.8 g of dipropylamine butane sulfonic acid inner salt obtained in the previous step and 9.8 g of sulfuric acid, react at 60° C. for 5 h, and after cooling, vacuum-dry to obtain [sulfuric acid] [dipropylamine 4- Butylammonium sulfonate] ionic liquid intermediate.
[0101] Under stirring conditions, 7.2 g of copper oxide and 32.2 g of the [sulfuric acid] [dipropylamine 4-butylammonium sulfonate] ionic liquid intermediate obtained in the previous step...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com