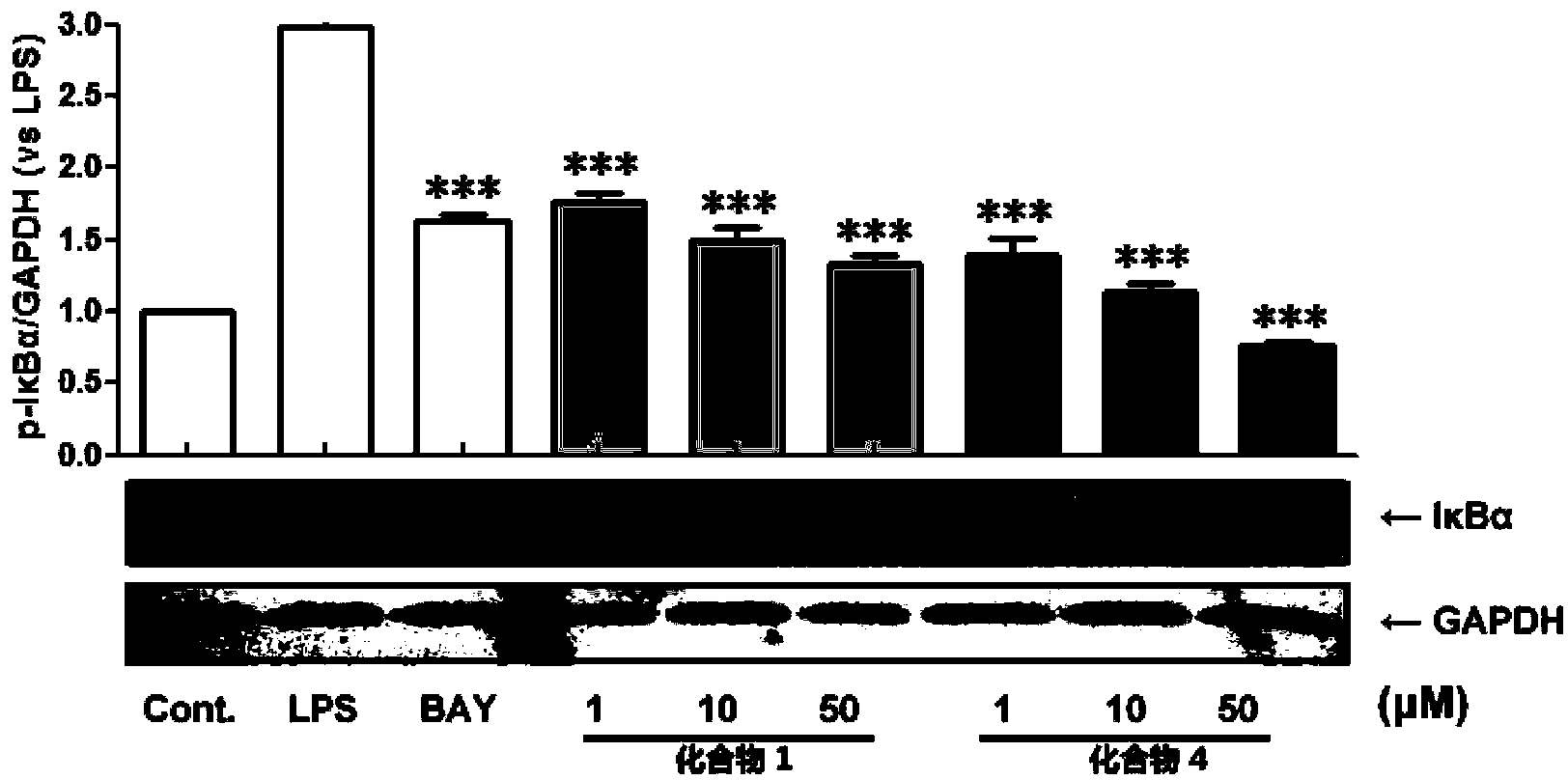
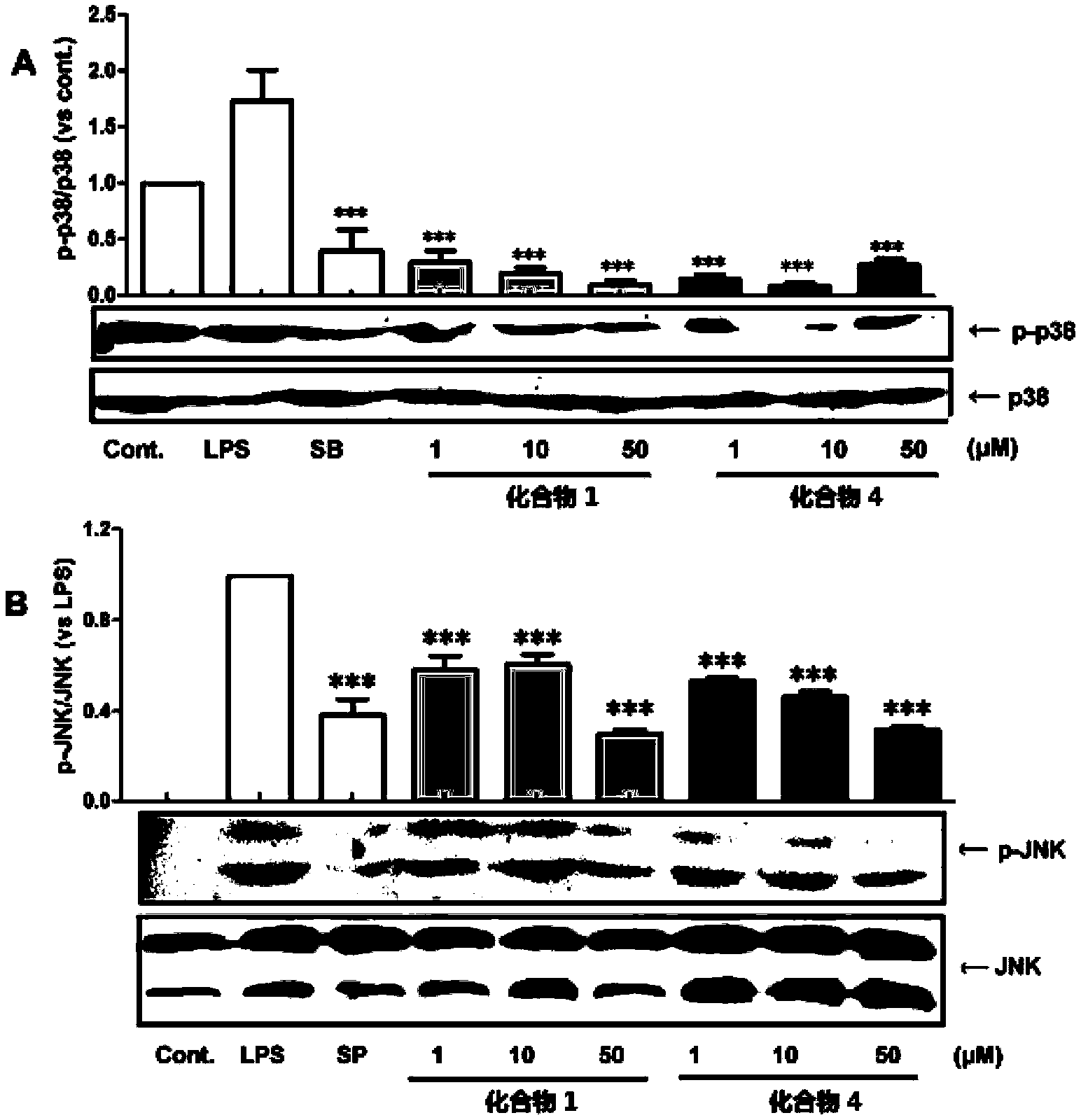
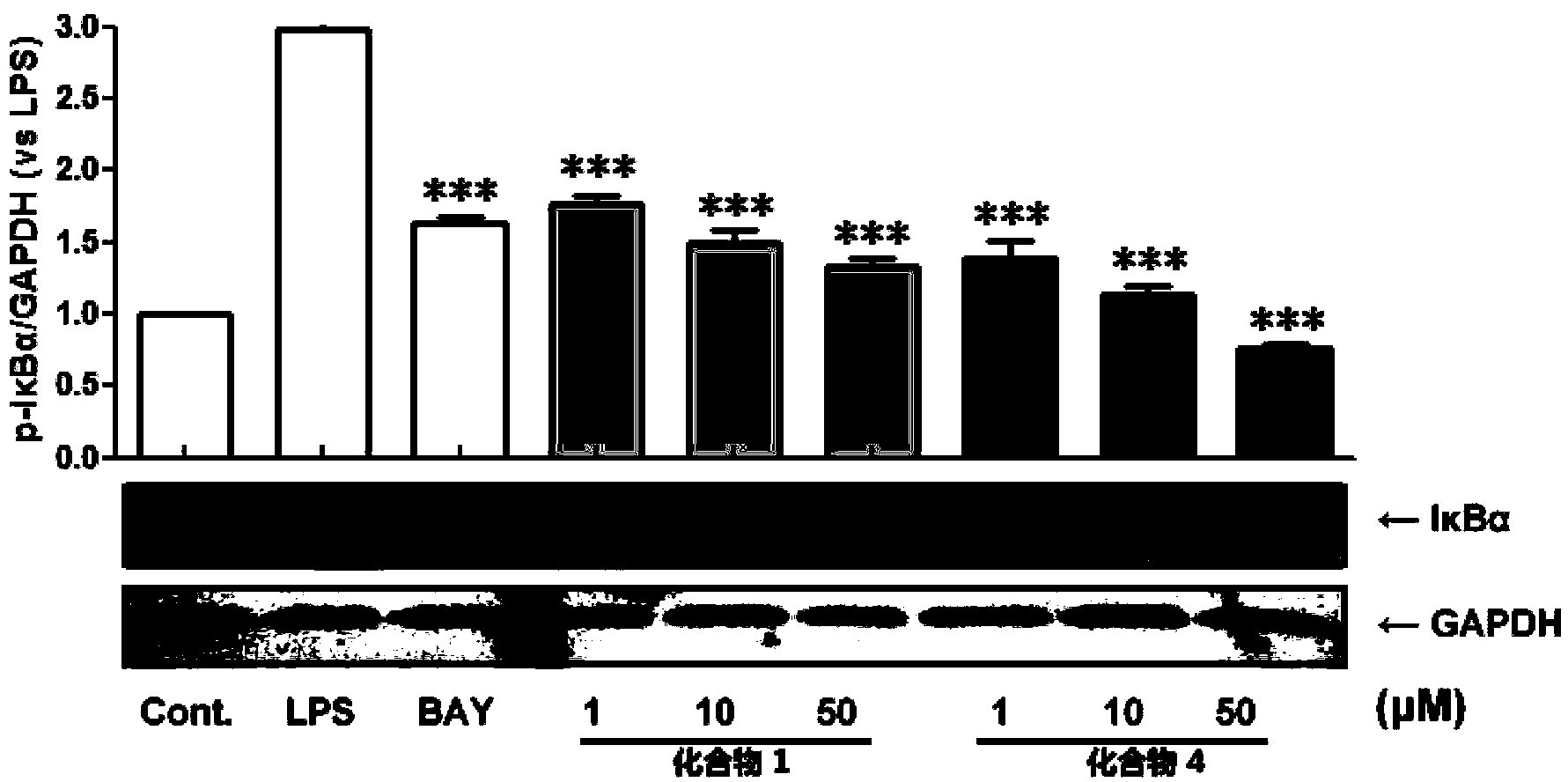
Phenanthrene and dihydrophenanthrene compounds and application thereof
A compound, the technology of hydroxydihydrophenanthrene, which is applied in the field of phenanthrene and dihydrophenanthrene compounds, can solve the problems of large toxic side effects and poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Extraction, separation and structural identification of compounds
[0031] 1.1 Instruments, reagents and fillers
[0032]UV1902 ultraviolet spectrophotometer; polarimetry using Perkin-Elmer 341 polarimeter; Perkin-Elmer FT-IR infrared spectrophotometer, KBr pellets; circular dichroism (CD) measurement using JASCO J-810; nuclear magnetic resonance experiment Bruker Avance 600 nuclear magnetic resonance instrument was used; mass spectrometry was determined using Bruker Daltonics Bio-TOF-Q type; high performance liquid chromatography: Agilent 1260 HPLC, and the preparative column used Kromasil 100-10-C18.
[0033] Methanol (analytical pure): Chengdu Kelong Chemical Reagent Factory; Chloroform (analytical pure): Chongqing Jiyuan Chemical Co., Ltd.; Ethyl acetate (analytical pure): Tianjin Jinkangde Technology Co., Ltd.; n-Butanol (analytical pure): Chengdu City Kelong Chemical Reagent Factory; petroleum ether (analytical pure): Tianjin Kangde Technology Co., Ltd...
Embodiment 2
[0081] Embodiment 2: the synthetic preparation of compound of the present invention
[0082] The general formula compound I of the present invention can be according to the following scheme figure 1 To prepare, wherein the substituent R 8 , R 9 , R 10 The substituents in the compounds of general formula I are as defined above.
[0083]
[0084] Add compound A (3.5mmol) and compound B (7mmol) into a 50mL two-necked bottle, connect it to a reflux condenser, pump air, and protect it with nitrogen. After adding acetic anhydride (20 mL) and triethylamine (5 mL) via needle injection, the mixture was reacted under reflux for 6 hours. After the reaction, the reaction solution was slowly poured into a beaker filled with a large amount of ice water, and kept stirring, at which point solids were precipitated. After the mixture was filtered, the resulting solid was recrystallized from ethanol to give compound C.
[0085] Compound C (5.56 mmol), copper powder (28.8 mmol) and quino...
Embodiment 3
[0100] Example 3: The NO production-inhibiting activity of the compounds of the present invention.
[0101] 3.1 Materials
[0102] Compound (1-33); mouse RAW264.7 macrophages; endotoxin (LPS 1 μg / mL); BAY (10 μM, positive control) NO detection kit (Beyotime, Haimen, China);
[0103] 3.2 Main Instruments
[0104] Fluorescent microplate reader (Thermo Scientific, Waltham, MA, USA)
[0105] 3.3 Method
[0106] 3.3.1 Measurement of NO production by LPS-stimulated RAW264.7 macrophages
[0107] Inoculate RAW264.7 macrophages in 96-well plate, inoculate 1×10 per well 4 After the cells were inoculated and attached to the wall, the compound to be tested (1-8) was added and incubated for 2 hours, and then LPS (final concentration: 1 μg / mL) was added and incubated for 24 hours. Finally, take the 96-well plate culture medium and use the NO kit to detect the NO content by measuring its OD 550 To represent. Three replicate wells were taken for each experiment, and the experiment was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com