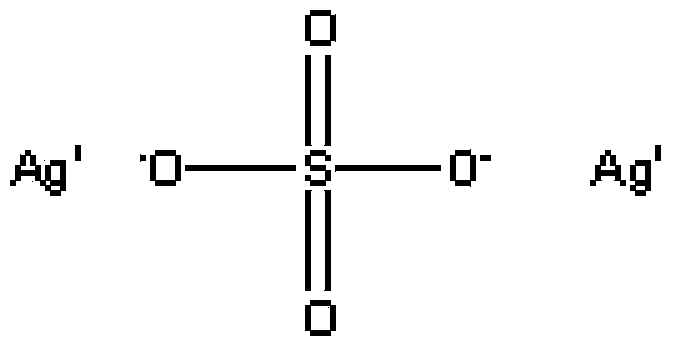
Method for synthesizing high-purity silver sulfate
A synthesis method and technology of silver sulfate, applied in the directions of silver compounds, silver compounds, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 10kg of silver nitrate to 10L of deionized water, stir to dissolve, filter, then add 21L of sodium hydroxide solution with a mass fraction of 10% to form a silver oxide precipitate, and finally add 0.275kg of purifying agent composition and stir for 2 hours, filter out the silver oxide precipitate , washed the precipitate twice with deionized water, drained and centrifuged, and gradually added to 16kg of concentrated sulfuric acid with a mass fraction of 95-98% and a temperature of 80°C to dissolve it in batches under stirring, and continued heating to 135°C , continue to keep warm for 3 hours, after cooling, slowly pour the solution into 13MΩ.CM deionized water to precipitate silver sulfate, then filter out silver sulfate, wash twice with 0.5L ethanol with a mass fraction of 99.5%, and then dry it. 8.39kg of high-purity grade silver sulfate, the yield is 91.39%, and the purity is 99.72%, NO3 -1 0.0009%.
[0021] Wherein, the above-mentioned cleaning agent composit...
Embodiment 2
[0023] Add 10kg of silver nitrate to 10.5L of deionized water, stir to dissolve, filter, then add 21.5L of sodium hydroxide solution with a mass fraction of 10% to form a silver oxide precipitate, and finally add 0.28kg of purifying agent composition and stir for 2.5h, filter out For silver oxide precipitation, wash the precipitate twice with deionized water, drain and centrifuge, gradually add it to 17kg of concentrated sulfuric acid with a mass fraction of 95-98% and a temperature of 85°C to dissolve it in batches under stirring, and continue heating To 139°C, keep warm for 3.5 hours, after cooling, slowly pour the solution into 13MΩ.CM deionized water to precipitate silver sulfate, then filter out silver sulfate, rinse twice with 0.65L ethanol with a mass fraction of 99.5% Dry to obtain 8.26kg of high-purity silver sulfate, the yield is 89.98%, the purity is 99.81%, NO3 -1 is 0.00087%.
[0024] Wherein, the scavenger composition mentioned above is a mixture of citric acid,...
Embodiment 3
[0026] Add 10kg of silver nitrate to 11L of deionized water, stir to dissolve, filter, then add 22L of sodium hydroxide solution with a mass fraction of 10% to form a silver oxide precipitate, and finally add 0.285kg of purifying agent composition and stir for 3 hours, filter out the silver oxide precipitate , washed the precipitate twice with deionized water, drained and centrifuged, and gradually added to 18kg of concentrated sulfuric acid with a mass fraction of 95-98% and a temperature of 90°C to dissolve it in batches under stirring, and continued heating to 142°C , continue to keep warm for 4 hours, after cooling, slowly pour the solution into 13MΩ.CM deionized water to precipitate silver sulfate, then filter out silver sulfate, wash twice with 0.8L ethanol with a mass fraction of 99.5%, and then dry it. High-purity grade silver sulfate 8.08kg, yield rate is 88.02%, and purity is 99.82%, NO3 -1 is 0.00085%.
[0027] Wherein, the above-mentioned cleaning agent compositio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com