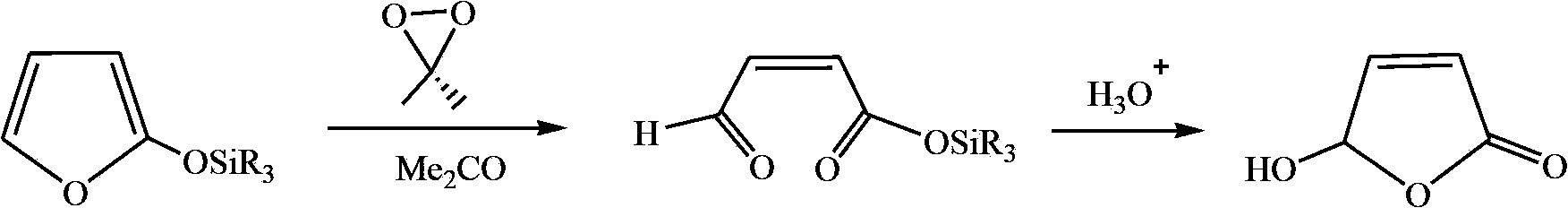
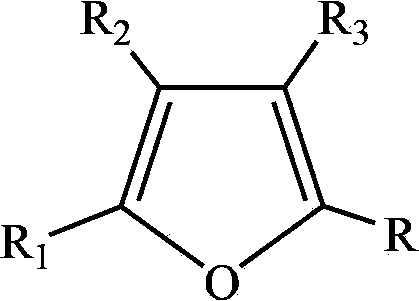
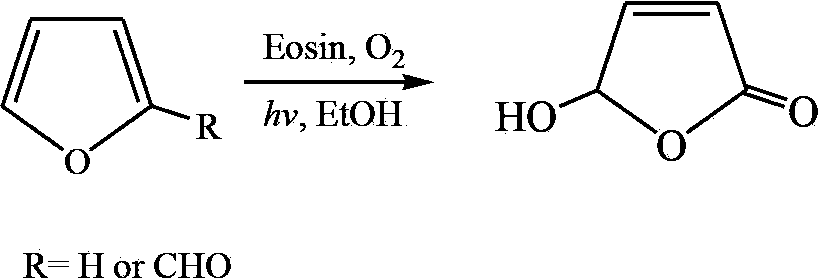Synthetic method of hydroxyl butenolide and congener thereof
A technology for synthesizing hydroxybutenoic acid lactone and hydroxybutenoic acid lactone, applied in organic chemistry and other directions, can solve the problems of low substrate concentration, pollution and high risk, achieve high yield, simplify purification steps, and cost cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example
[0032] (1) Preparation of rose bengal dyeing solution: accurately weigh 1.0 g of rose bengal dye and 1.0 L of distilled water to prepare 1.0 g / L rose bengal dyeing solution.
[0033] (2) Dyeing process: Take 7.3g of treated wool, weigh 100ml of 1.0g / L rose red dye solution with a measuring cylinder, pour it into the dyeing bottle to dilute to 200ml, and adjust the pH to 3-4 with 1mol / L glacial acetic acid; After the dyeing bath is configured, the temperature is raised to 40°C, and the wool soaked in 50°C water for 15 minutes is squeezed dry and put into the dyeing bath to start dyeing. The dyeing bath is gradually heated up to 100°C at a rate of 2°C / min, and dyed at 100°C for 60 minutes. After dyeing, the wool is taken out, washed with distilled water until the wool no longer fades, and then vacuum-dried at 40°C.
[0034] (3) Acetonitrile treatment: Measure 50ml of acetonitrile and pour it into a 250ml three-necked flask, put the wool treated in step (2) into the three-necked ...
Embodiment 2
[0038] Preparation of wool-loaded rose red photosensitizer and its application in photocatalytic oxidation of furfural to prepare butenolide: (different polar solvents)
[0039] (1) Preparation of rose bengal dyeing solution: accurately weigh 1.0 g of rose bengal dye and 1.0 L of distilled water to prepare a 1.0 g / L rose bengal dyeing solution.
[0040] (2) Dyeing process: Take 7.3g of treated wool, weigh 100ml of 1.0g / L rose red dye solution with a measuring cylinder, pour it into the dyeing bottle to dilute to 200ml, and adjust the pH to 3-4 with 1mol / L glacial acetic acid; After the dyeing bath is configured, the temperature is raised to 40°C, and the wool soaked in 50°C water for 15 minutes is squeezed dry and put into the dyeing bath to start dyeing. The dyeing bath is gradually heated up to 100°C at a rate of 2°C / min, and dyed at 100°C for 60 minutes. After dyeing, the wool is taken out, washed with distilled water until the wool no longer fades, and then vacuum-dried at...
Embodiment 3
[0045] Preparation of silk-loaded rose bengal photosensitizer and its application in photocatalytic oxidation of furoic acid to prepare butenolide:
[0046] (1) Preparation of rose bengal dyeing solution: accurately weigh 1.0 g of rose bengal dye and 1.0 L of distilled water to prepare a 1.0 g / L rose bengal dyeing solution.
[0047] (2) Dyeing process: Take 5.0g of the treated silk, weigh 100ml of 1.0g / L rose red dye solution with a measuring cylinder, pour it into the dyeing bottle and dilute to 200ml, and adjust the pH to 3-4 with 1mol / L glacial acetic acid; After the dyeing bath is configured, the temperature is raised to 40°C, and the silk soaked in 50°C water for 15 minutes is squeezed dry and put into the dyeing bath to start dyeing. The dyeing bath is gradually heated up to 100°C at a rate of 2°C / min, and dyed at 100°C for 60 minutes. After dyeing, the silk is taken out, washed with distilled water until the silk no longer fades, and dried in vacuum at 40°C.
[0048] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com