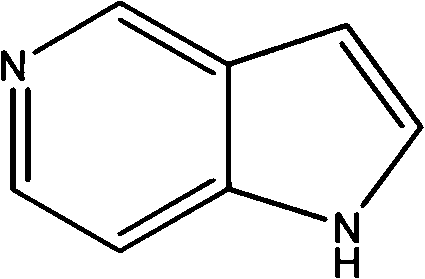
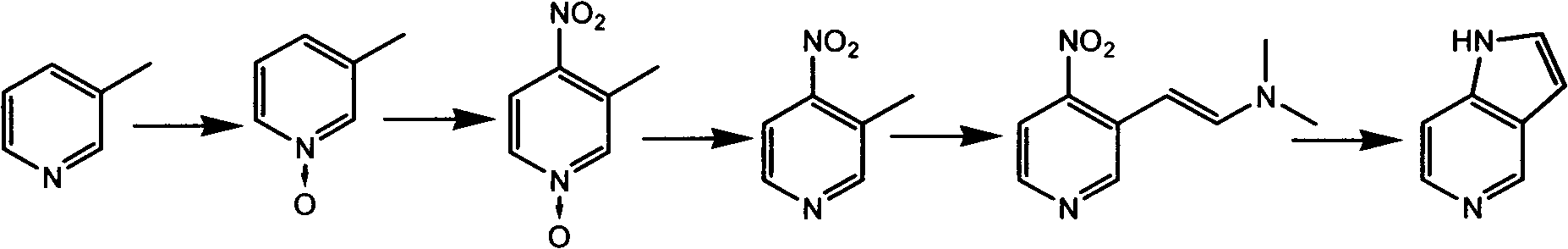
5-azaindole preparation method
The technology of an azaindole and a production method is applied in the field of preparing 5-azaindole, can solve the problems such as inability to apply enlarged production, low reaction temperature, etc., and achieves low production cost, mild chemical reaction conditions, and strong operability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be described in detail below through specific embodiments, but the present invention is not limited to these examples.
[0025] Preparation of 3-picoline nitrogen oxide: 3-picoline (188.3g, 2.0mol) was added to 800ml of glacial acetic acid, and under stirring, 350ml of 35% hydrogen peroxide was slowly added dropwise to the reactor. After the dropwise addition, the mixture was heated to 80° C., and after 7 hours of reaction, glacial acetic acid and excess hydrogen peroxide were removed by rotary evaporation to obtain 220 g of crude 3-picoline nitrogen oxide. It was used directly in the next step without purification.
[0026] Preparation of 3-methyl-4-nitropyridine nitrogen oxide: below 20°C, slowly drop 500ml of nitric acid into 650ml of concentrated sulfuric acid to prepare the mixed acid required for the reaction. Add 3-picoline nitrogen oxide (220 g, 2.0 mol) dropwise to the mixed acid prepared above, and control the dropping rate so that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com