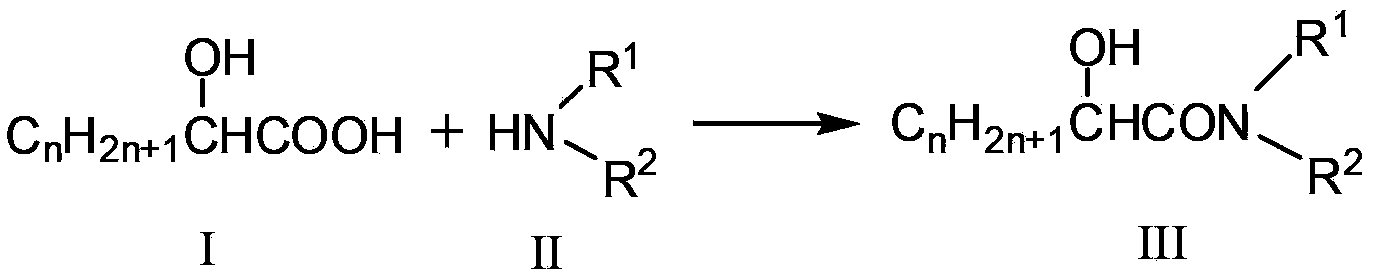
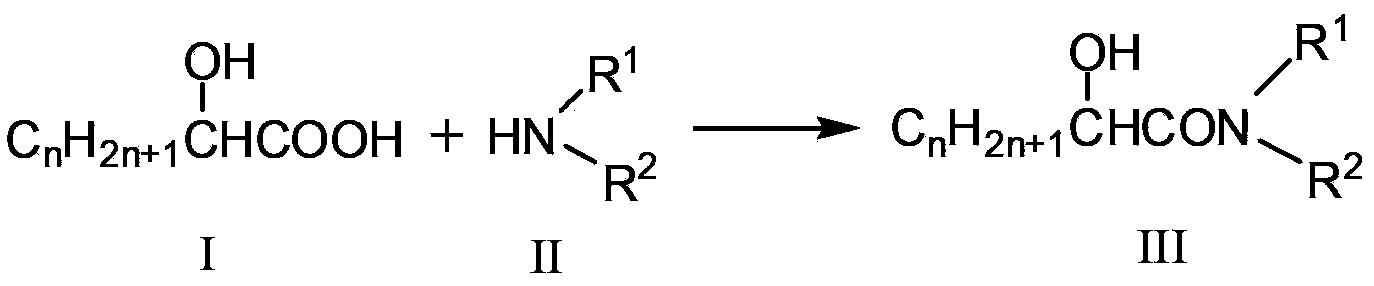
Synthetic method for N, N-disubstituted-2-hydroxyl aliphatic amide compound
A technology for amide compounds and synthesis methods, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid amides, etc., can solve the problems of difficulty in microwaves, affect the reaction effect and yield, etc., and achieves short reaction routes and raw materials. Simple, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 0.2mol glycolic acid in 150mL methanol, and 0.25mol dimethylamine gas in 50mL methanol, pour the two solutions into a high-pressure batch reactor, and then feed N into the autoclave. 2 , the pressure is 2.4MPa. The reaction was carried out for 6 hours at a rotation speed of 900 rpm and a temperature of 150°C. After the reaction, wait for the temperature in the kettle to drop to room temperature, and distill under reduced pressure to obtain 3.68 g of N,N dimethylglycolamide red solution with a purity of 84.4% and a yield of 15.1%. Characterization data of the purified product: melting point 44°C; 1 H NMR (CDCl 3 )δ=2.81(s,3H),2.94(s,3H),3.48(s,1H,OH),4.05(s,2H); MS(EI):103[M] + .
Embodiment 2
[0029] Dissolve 0.213mol of glycolic acid in 150mL of methanol, and 0.681mol of dimethylamine gas in 50mL of methanol, pour the two solutions into a high-pressure batch reactor, and add Al 2 o 3 powder, and N into the autoclave 2 , the pressure is 2.4MPa. The reaction was carried out for 6 hours at a rotation speed of 900 rpm and a temperature of 150°C. After the reaction was completed, wait until the temperature in the kettle dropped to room temperature, open the kettle and distill the obtained solution under reduced pressure to obtain 12.369 g of white crystals of N,N-dimethylglycolamide with a purity of 96.5% and a yield of 57.42%.
Embodiment 3
[0031] Dissolve 0.214mol of glycolic acid in 150mL of methanol, 0.854mol of dimethylamine gas in 50mL of methanol, pour the two solutions into a high-pressure batch reactor, and add 3% of the molar amount of glycolic acid MoO 3 , and then N into the autoclave 2 , the pressure is 2.4MPa. The reaction was carried out for 5 hours at a rotation speed of 900 rpm and a temperature of 150°C. After the reaction was completed, wait until the temperature in the kettle dropped to room temperature, open the kettle and distill the obtained solution under reduced pressure to obtain 13.235 g of white crystals of N,N-dimethylglycolamide with a purity of 99.4% and a yield of 59.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com