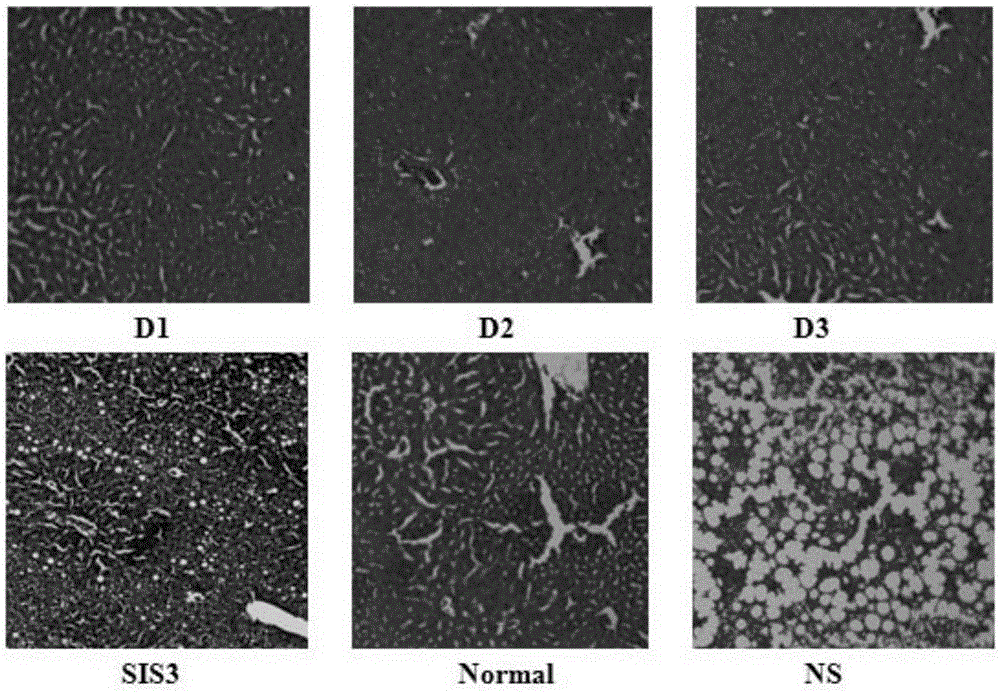
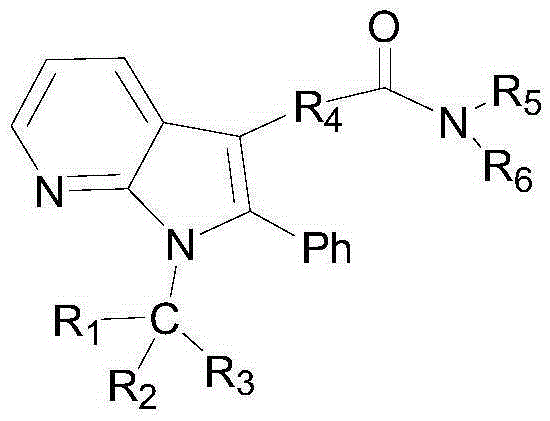
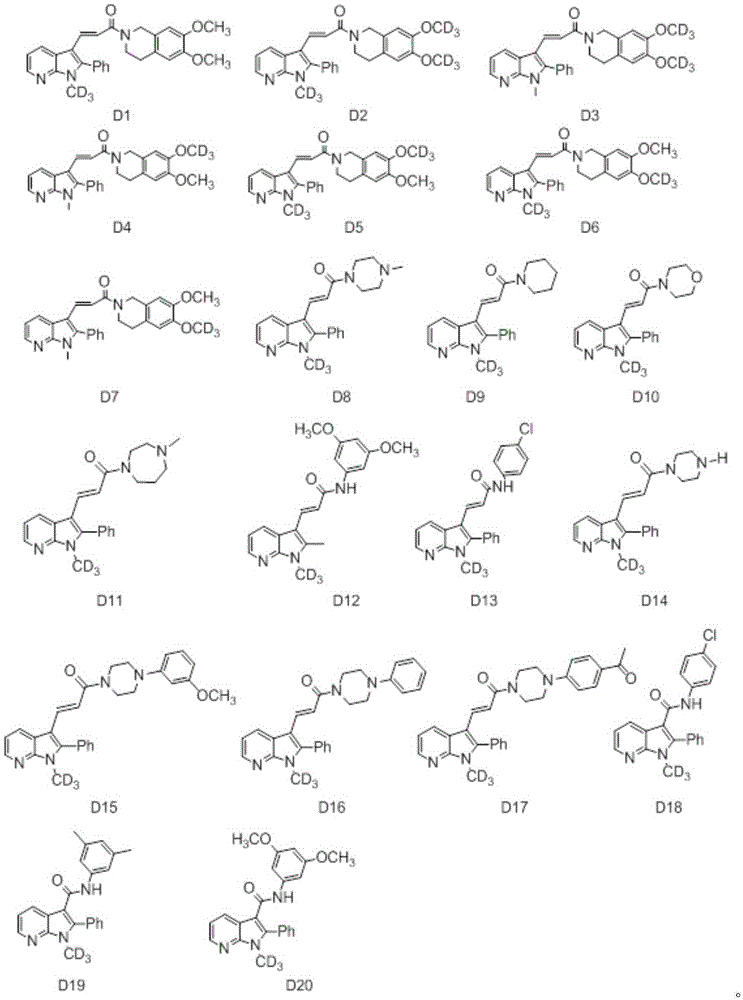
A compound for treating diabetes
A compound and composition technology, applied in the field of treating diabetes compounds, can solve problems such as allergies, and achieve the effects of improving body weight, reducing toxic and side effects, improving blood lipid indexes and oral glucose tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of embodiment 1 compound D1
[0032]
[0033] Synthesis of Compound 2
[0034] N 2 Under protection, DMF was added to a three-necked flask containing 5 g of compound 1 and 1.25 g of NaH, stirred at 0°C for 10 min, and then slowly added CD 3 I, stirred overnight at room temperature. TLC monitoring, after the reaction was completed, slowly added water dropwise to the reaction flask, then extracted with EA, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate, concentrated and then column chromatographed to obtain 3.95 g of a light yellow solid with a yield of 78.9%. . 1 HNMR (400MHz, CDCl 3 ) 8.36 (t, 1H), 7.92 (q, 1H), 7.57–7.42 (m, 5H), 7.11–7.07 (m, 1H), 6.52 (s, 1H).
[0035] Synthesis of compound 3
[0036] N 2 Under protection, after dissolving 3.94g of compound 2 in DMF, slowly add 2.4mL of POCl dropwise at 0°C 3 , After stirring for 3 h, the reaction was monitored by TLC. After the reaction of the raw materia...
Embodiment 2
[0041] The synthesis of embodiment 2 compound D2
[0042]
[0043] Synthesis of compound 7
[0044] Under nitrogen protection, DMF was added to a three-necked flask containing 5 g of compound 1 and 1.25 g of NaH, stirred at 0°C for 10 min, and then CH was slowly added dropwise. 3 I, stirred overnight at room temperature. After the reaction was completed, water was slowly added dropwise to the reaction flask, and then extracted with EA. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and then column chromatographed to obtain about 4.05 g of a light yellow product with a yield of 79.5%.
[0045] Synthesis of Compound 8
[0046] Under nitrogen protection, after dissolving 4.05g of compound 7 in DMF, slowly add 2.4mL of POCl dropwise at 0°C3 , After stirring for 3 h, the reaction was monitored by TLC. After the reaction of the raw materials was complete, 50 mL of ice water was added to the reaction flask, and the pH was ad...
Embodiment 3
[0053] The synthesis of embodiment 3 compound D3
[0054]
[0055] Synthesis of Compound 13
[0056] Take 28.1mg (0.1mmol) compound 4, 27mg (0.2mmol) HOBT, 57.5mg (0.3mmol) EDCI, dissolve in 2mL DMF, add 0.05mL triethylamine, stir at room temperature for 30 minutes; then add 29.5mg (0.12mmol) 1,2,3,4-Tetrahydro-6,7-isoquinoline diol hydrobromide (10), stirred overnight at room temperature. TLC monitoring, after the completion of the reaction, add water, extract with EA three times (15 mL each time), wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, concentrate and directly use in the next reaction.
[0057] Synthesis of Compound 14 (D3)
[0058] in N 2 Under protection, dissolve the above crude product and appropriate amount of NaH in DMF, stir at 0°C for 30 minutes, then slowly add CD 3 I, reaction at room temperature. After the reaction was complete, water was slowly added dropwise to the reaction flask, and then extracted with EA. The o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com