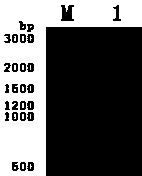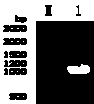Application of Bostrycin to preparation of protein tyrodine phosphatase (PTP) bonding agents or inhibitors
A tyrosine phosphatase binder and tyrosine phosphatase technology, which is applied in the field of medicine, can solve the problems of weakened signal pathways and the inability of cells to respond to it, and achieve good therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
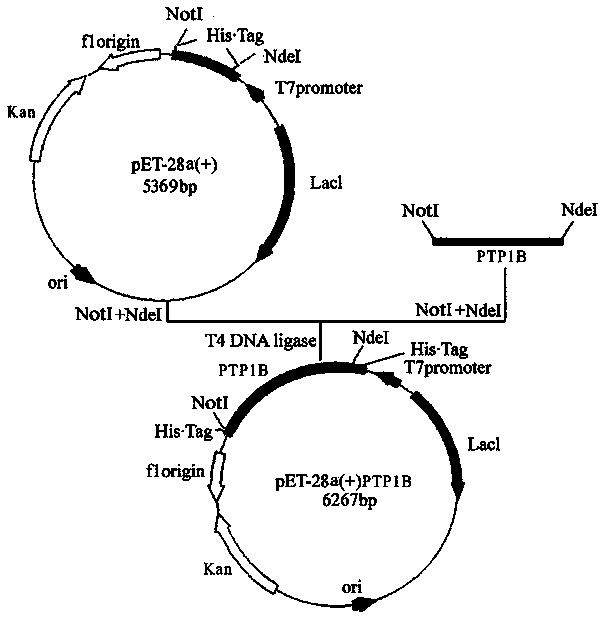
[0125] Fusion expression of human tyrosine phosphatase 1B protein
[0126] (1). The gene cloning of human tyrosine phosphatase 1B is carried out according to the following steps:
[0127] According to the known cDNA sequence of human tyrosine phosphatase 1B, two primers were designed and synthesized (synthesized by Shanghai Yingwei Jieji Trading Co., Ltd.), and the upstream primers were:
[0128] 5’ GGATC CATATG ATGGAGATGGAAAAGGAGTTCGAGC3';
[0129] Downstream primers are:
[0130] 5' AATAT GCGGCCGC ATTGTGTGGCTCCAGGATTCGTTT3'; Add NdeI and NotI restriction sites (underlined parts) to the 5' ends of the upstream and downstream primers, respectively. Using the purchased cDNA cloning vector of human tyrosine phosphatase 1B (purchased from Beijing Yiqiao Shenzhou Biotechnology Co., Ltd.) as a template, use the above two primers to amplify 963 base pairs of human tyrosine by PCR DNA fragment of phosphatase 1B. The PCR reaction conditions are: 0.05 μg of cDNA cloning vector...
Embodiment 2
[0139] The purification of expressing recombinant human tyrosine phosphatase 1B fusion protein comprises the following steps:
[0140] (1). The engineering bacterium expressing human tyrosine phosphatase 1B fusion protein obtained in Example 1 was optimized for protein induction expression: the engineering bacterium expressing human tyrosine phosphatase 1B fusion protein obtained in Example 1 was inoculated into 5 ml containing kanamycin In a test tube of liquid LB culture medium (50 μg / ml), shake culture at 37°C overnight. Then explore the effects of inducer IPTG concentration, induction temperature and induction time on protein production. The above culture was transferred to fresh LB medium containing 50 μg / mL kanamycin at a ratio of 1:100, and cultured with shaking at 200 rpm at 37°C until OD600=0.6. IPTG concentration optimization: add IPTG to the culture to a final concentration of 0.1mM~0.8mM, and continue shaking culture for 4 hours; induction temperature optimizatio...
Embodiment 3
[0147] Identification of Purified Proteins by Western Blot
[0148] SDS-PAGE of ultrafiltration-purified human tyrosine phosphatase 1B fusion protein: take 5 μl of purified ultrafiltration-concentrated human tyrosine phosphatase 1B fusion protein, mix with 2 μl of loading buffer (Thermo company product), boil water Bath for 10 minutes, after cooling, centrifuge and load the sample. Cut filter paper and PVDF membrane of appropriate size, and soak them in transfer buffer and methanol respectively to fully infiltrate them. Put the filter paper, PVDF membrane, and SDS-PAGE glue into the transfer membrane tank in order, and the constant current is 200mA, and the time is 2 hours. The membrane was taken out and placed in blocking solution, and blocked at room temperature for 2 hours. The blocking solution was discarded, and the membrane was washed 3 times with TBST, 10 min each time. Mouse anti-histidine tag (product of Sigma Company) at an appropriate dilution (1:3000) was used a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com