Preparation method of molybdenum hexafluoride
A technology of molybdenum hexafluoride and hydrogen fluoride gas, applied in the direction of molybdenum halide, etc., can solve the problems of difficult reaction conditions, complicated reaction process, complex reaction, etc., and achieve the effect of easy control of reaction conditions, simple reaction process and high purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
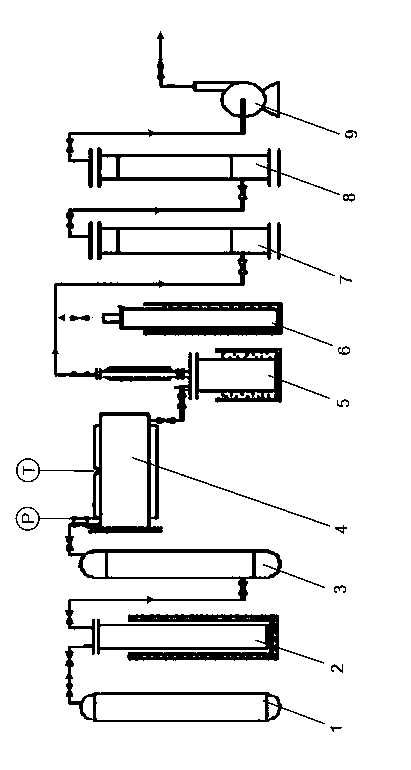
[0033] Such as figure 1 Shown, a kind of preparation method of molybdenum hexafluoride comprises the following steps:
[0034] (i) Fluorine gas purification
[0035] The fluorine (F) in the fluorine buffer tank 1 2 ) into the cooling tower 2, cooled to -70~-90°C, first remove a large amount of hydrogen fluoride (HF) gas in the fluorine gas, and then pass the fluorine gas containing a small amount of hydrogen fluoride into the absorber equipped with sodium fluoride (NaF) In tower 3, a small amount of hydrogen fluoride remaining in the fluorine gas is removed at 100°C,
[0036] (ii) Synthesis reaction
[0037] Put the metal molybdenum in the reactor 4, preheat to 200°C, vacuumize the reactor 4 to -0.1~-0.09MPa, slowly introduce the purified fluorine gas, and the fluorine gas and the metal molybdenum contact in the reactor 4 Synthesis reaction occurs, the reaction temperature of reactor 4 is maintained at 200-350°C, the reaction time is 2-4 hours, the pressure is maintain...
Embodiment 1
[0045] 50g of metal molybdenum is placed in the reactor 4, and after the reactor is sealed and tested for leaks, it is preheated to 200°C, the heating is stopped, and the reactor 4 is evacuated to -0.1Mpa. The purified fluorine gas is slowly introduced into the reactor 4, and the metal molybdenum and the fluorine gas synthesize and react to generate molybdenum hexafluoride. The temperature of the reactor 4 is controlled at 200° C., and the pressure is maintained at -0.05 MPa. The reaction ends after 2 hours.
[0046] The crude molybdenum hexafluoride product from the reactor 4 enters the collector 5, and the temperature of the collector 5 is -50°C. The light component impurities such as oxygen and nitrogen in the molybdenum hexafluoride enter the tail gas treatment system, and the distillation of the collector 5 The temperature rises to 40°C, the temperature of the reflux column reaches 35°C, the temperature of the product steel cylinder 6 is maintained at 0°C, and the product ...
Embodiment 2
[0048] 100g of metal molybdenum is placed in the reactor 4, and after the reactor 4 is sealed and tested for leaks, it is preheated to 200°C, the heating is stopped, and the reactor 4 is evacuated to -0.05Mpa. The purified fluorine gas is slowly introduced into the reactor 4, and the metal molybdenum and the fluorine gas synthesize and react to form molybdenum hexafluoride. The temperature of the reactor 4 is controlled at 280° C., and the pressure is maintained at -0.08 MPa. After 3 hours, the reaction ends.
[0049] The crude molybdenum hexafluoride product from the reactor 4 enters the collector 5, and the temperature of the collector 5 is -25°C. The light component impurities such as oxygen and nitrogen in the molybdenum hexafluoride enter the tail gas treatment system, and the distillation of the collector 5 The temperature rises to 40°C, the temperature of the reflux column reaches 35°C, the temperature of the product steel cylinder 6 is maintained at -25°C, and the produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com