Metformin hydrochloride dry suspension and preparation method thereof
A kind of technology of dry metformin hydrochloride and metformin hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
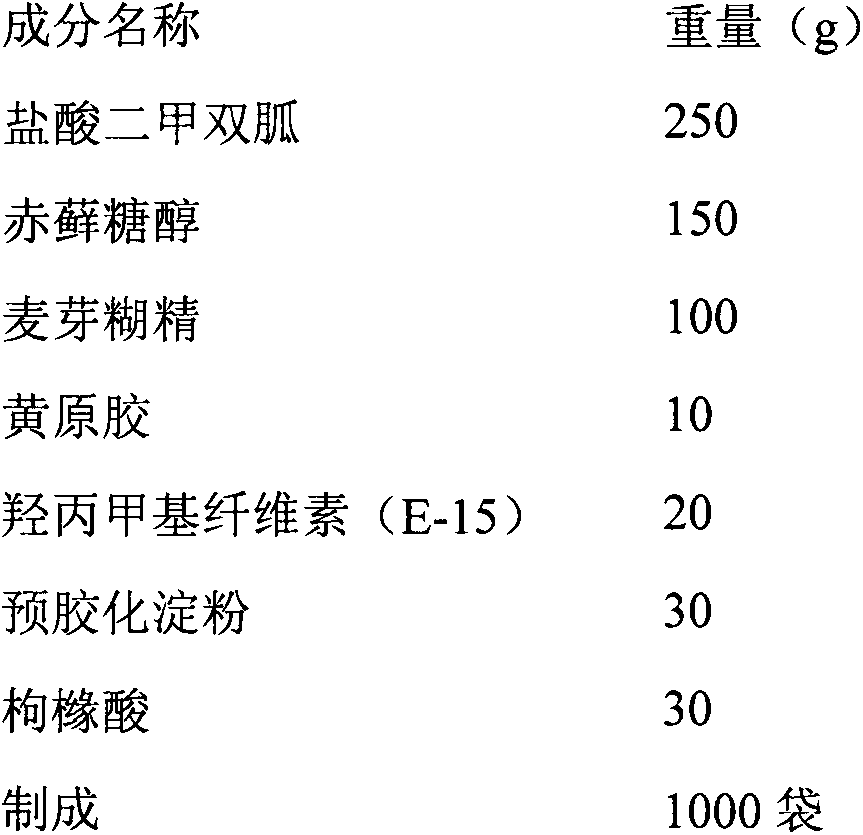
[0022] Each bag contains 250mg of metformin hydrochloride, and its prescription is:
[0023]
[0024] The preparation process is as follows:
[0025] Pass the metformin hydrochloride and the above-mentioned excipients through an 80-mesh sieve, then mix them uniformly, add an appropriate amount of 3% hydroxypropylmethyl cellulose (E-15)-60% ethanol solution to granulate, dry at 50℃, and use a 40-mesh sieve Sizing to obtain a dry suspension of metformin hydrochloride.
[0026] Evaluation: Weigh 1g of dry suspension of metformin hydrochloride and add 20g of hot water to make it uniformly dispersed. The particle size is large, the sedimentation is fast at the beginning, and the suspension is better after 30 minutes. The taste is bitter and has peculiar smell.
Embodiment 2
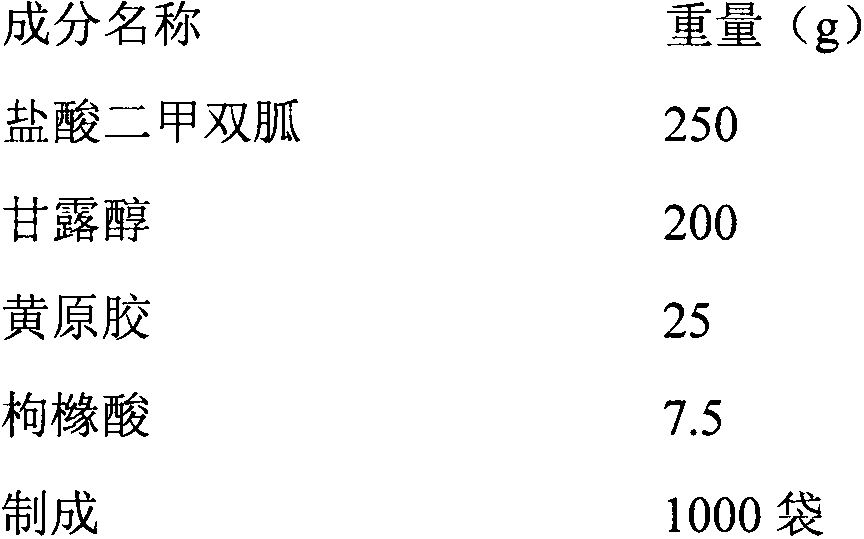
[0028] Each bag contains 250mg of metformin hydrochloride, and its prescription is:
[0029]
[0030] The preparation process is as follows:
[0031] Pass the metformin hydrochloride and the above-mentioned excipients through an 80-mesh sieve, then mix them evenly, add an appropriate amount of 3% hydroxypropylmethyl cellulose (E-15)-60% ethanol solution to granulate, dry at 50℃, use a 40-mesh sieve Sizing to obtain a dry suspension of metformin hydrochloride.
[0032] Evaluation: Weigh 1g of dry suspension of metformin hydrochloride and add 20g of hot water to make it uniformly dispersed. The particle size is large, the suspension state is not good, the taste is bitter, and there is a peculiar smell.
Embodiment 3
[0034] Each bag contains 250mg of metformin hydrochloride, and its prescription is:
[0035]
[0036] The preparation process is as follows:
[0037] The metformin hydrochloride and the above-mentioned auxiliary materials are passed through an 80-mesh sieve and mixed uniformly to prepare a dry suspension of metformin hydrochloride.
[0038] Evaluation: Weigh 1g of dry suspension of metformin hydrochloride and add 20g of hot water to make it evenly dispersed. The suspension state is not good, the sedimentation is relatively fast, the taste is bitter, and there is a peculiar smell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com