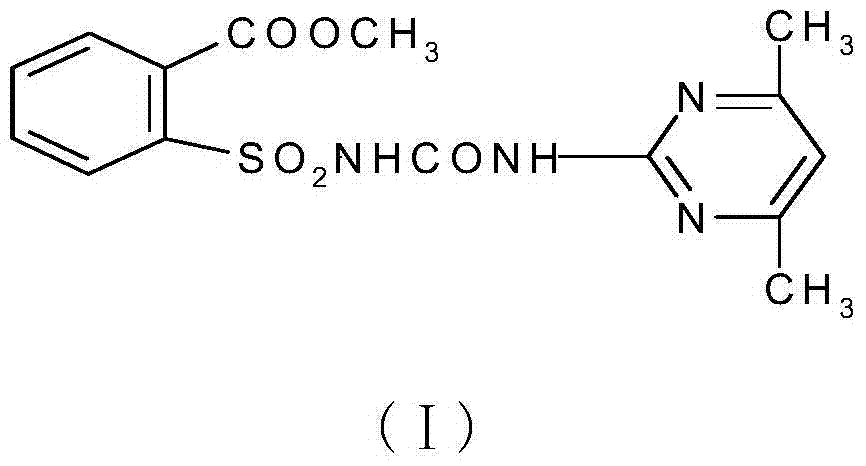
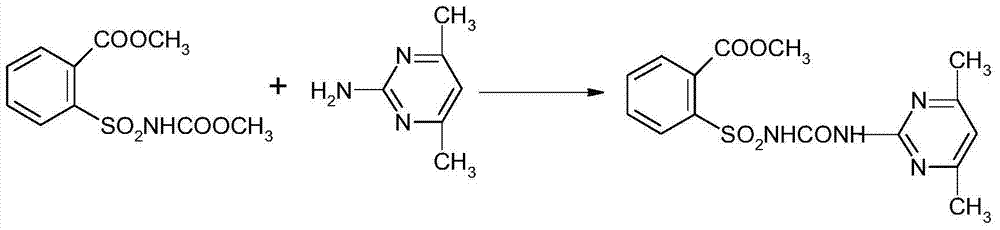
Synthetic method of 2-(4, 6-dimethyl pyrimidine-2-base amino formyl amino sulfonyl) methyl benzoate
A technology of methyl carbamoylaminosulfonyl and o-methoxycarbonylbenzenesulfonamidomethyl formate, applied in the field of synthesis of 2-benzoic acid methyl ester, can solve the problem of easy hydrolysis and deterioration, hidden dangers of environmental protection and safety, environmental pollution, etc. problems, to achieve the effect of easy control of reaction conditions, avoidance of safety accidents, and reduction of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
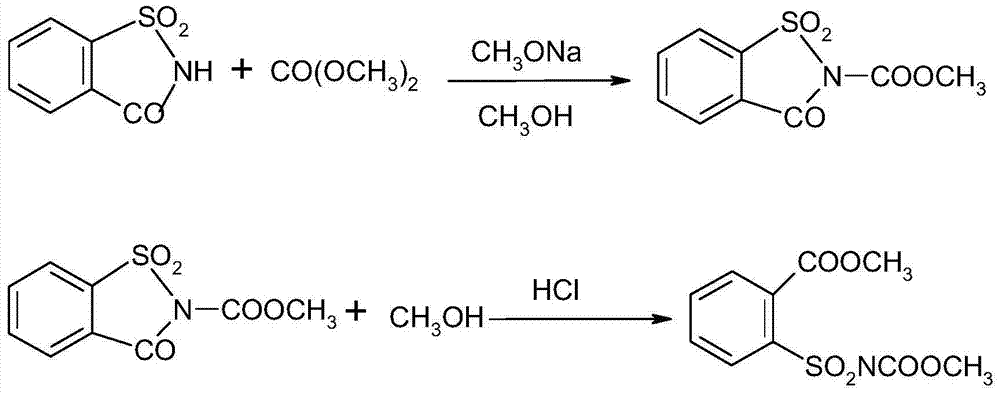
[0040] (1) First, add saccharin (20g, 0.11mol), sodium methoxide (21.6g, 0.12mol) and methanol 100ml into a 250mL three-neck flask, heat to 70-75°C, and then dropwise add dimethyl carbonate (10.8g, 0.12mol) reflux reaction for 8.5h, then distilled off the solvent, lowered the temperature to below 40°C and added 80ml of water, and finally neutralized to neutral with 5% hydrochloric acid by mass percentage, filtered and dried to obtain 24.3g of N-methyl formate-phthalic acid Acylsulfonimide, content 98%, yield 90.0%.
[0041] IR (cm -1 ): 3090, 1793, 1742, 1588, 1362, 1187;
[0042] 1 H NMR (δppm): 3.63 (3H, s), 7.61 (1H, t), 7.85 (1H, t), 8.02 (1H, d), 8.41 (1H, d);
[0043] (2) Add 100ml of methanol, N-methyl formate-o-benzoylsulfonimide (12.1g, 0.05mol) and hydrochloric acid (5.0g) into a three-neck flask, heat to reflux for 1.5h, concentrate under reduced pressure and evaporate Methanol, washed with water until neutral, filtered, and dried to obtain 12.5 g of methyl o-me...
Embodiment 2
[0051] The difference between this embodiment and embodiment 1 is that steps (1) and (2) are combined:
[0052] Add saccharin (20g, 0.11mol), sodium methoxide (21.6g, 0.12mol) and methanol 100ml into a 250mL three-neck flask, heat to 70-75°C, then add dropwise dimethyl carbonate (10.8g, 0.12mol) to reflux reaction After 8.5h, the solvent was evaporated to dryness, 80ml of methanol and hydrochloric acid (2.5g) were added under cooling, and the reaction was heated to reflux for 1.5h. The methanol was evaporated by concentration under reduced pressure, washed with water, filtered, and dried to obtain 24.4g of o-methoxycarbonylbenzenesulfonamide Methyl formate, content 95%, yield 85%.
Embodiment 3
[0054] The difference between this example and Example 1 is that catalyst A in step (1) is sodium hydroxide 6g (0.15mol), and the yield of N-methyl formate-o-benzoylsulfonimide in step (1) 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com