Stilbene fluorescent whitening agent, and preparation method and application thereof
A technology of fluorescent whitening agent and stilbene, which is applied in the direction of styrene-based dyes, chemical instruments and methods, and addition of luminescent/fluorescent substances. Advanced problems, to achieve the effect of strong fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
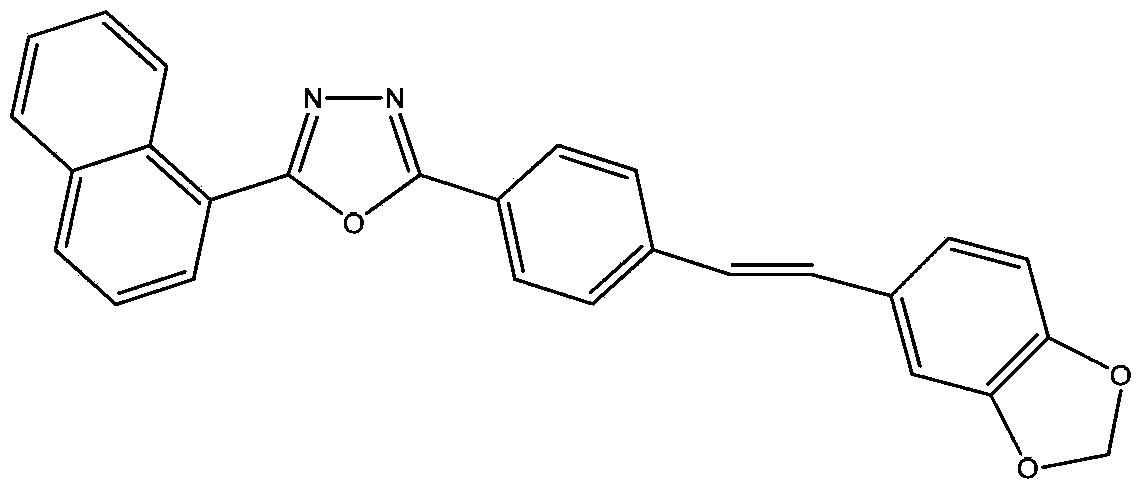
[0024] Synthesis of embodiment 11,3,4-oxadiazole stilbene fluorescent whitening agent (I)
[0025]
[0026] (1) Dissolve 0.0088mol 2-naphthyl-5-(4-(bromomethyl)phenyl)-1,3,4-oxadiazole in 0.0132mol triethyl phosphite, heat to 130°C and stir Refluxing reaction for 5 hours, after decompressing to distill off excess triethyl phosphite in the reaction system, add 5ml of n-hexane with stirring while hot to precipitate a solid product, and then recrystallize with a 1:1 mixture of tetrahydrofuran and n-hexane to obtain 3.32g of 4-( 5-Naphthyl-1,3,4-oxadiazol-2-yl)benzylphosphonate, yield: 89.4%. Melting point: 139-140°C. H NMR structural characterization data: 1 H NMR (400MHz, CDCl 3 )δ9.30(d,J=8.6Hz,1H,naphthalene-H),8.29(d,J=7.2Hz,1H,naphthalene-H),8.18(d,J=7.9Hz,2H,C 6 h 4 ,2,6-H),8.07(d,J=8.2Hz,1H,naphthalene-H),7.97(d,J=8.1Hz,1H,naphthalene-H),7.73(t,J=7.7Hz,1H ,naphthalene-H),7.63(t,J=7.6Hz,2H,naphthalene-H),7.53(d,J=7.9Hz,2H,C 6 h 4 ,3,5-H),4.08(q,J=7.2Hz,4H,CH 2 )...
Embodiment 2
[0029] Synthesis of 1,3,4-oxadiazole stilbene fluorescent whitening agent (I)
[0030] (1) Dissolve 0.0088mol 2-naphthyl-5-(4-(bromomethyl)phenyl)-1,3,4-oxadiazole in 0.0097mol triethyl phosphite, heat to 125°C and stir Reflux the reaction for 3 hours, distill off the excess triethyl phosphite in the reaction system under reduced pressure, add 5ml of n-hexane to precipitate the solid product while stirring while hot, and recrystallize with a 1:4 mixture of tetrahydrofuran and n-hexane to obtain 2.98g of 4-( 5-Naphthyl-1,3,4-oxadiazol-2-yl)benzylphosphonate, yield: 80.2%.
[0031](2) Dissolve 0.0020mol of 4-(5-naphthyl-1,3,4-oxadiazol-2-yl)benzylphosphonate and 0.0020mol of jasmonal in 10ml of N,N-dimethyl Formamide, and then dropwise add 4ml of anhydrous ethanol solution of potassium tert-butoxide with a mass ratio of 15% to the solution, stir and react at 60°C for 5h to obtain a reaction solution, cool and filter, and the volume ratio of the obtained solid is 2: The mixture...
Embodiment 3
[0033] 1. Determination of UV light of 1,3,4-oxadiazole stilbene fluorescent whitening agent
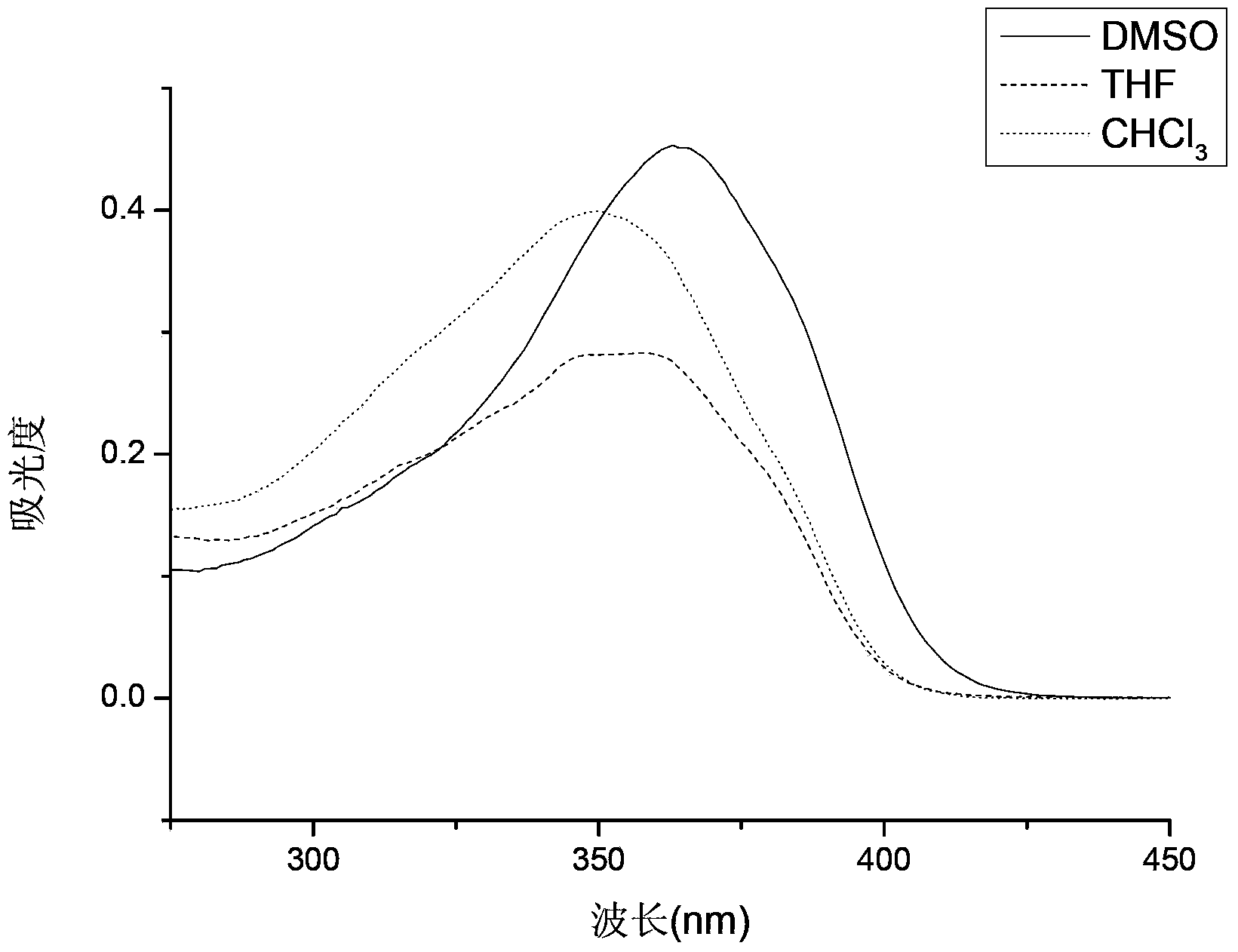
[0034] Compound (I) was mixed with solutions of different polarities (DMSO, THF, CHCl 3 ) to the same concentration of 1×10 -5 mol / L solution, take the wavelength range of 200-500nm to measure the ultraviolet absorption on the ultraviolet-visible spectrophotometer, and obtain its ultraviolet-visible absorption spectrum ( figure 1 ).
[0035] From figure 1 It can be seen from the figure that the maximum ultraviolet absorption wavelengths of fluorescent whitening agents in different polar solutions are 363nm, 358nm and 350nm respectively, and the red shift occurs with the increase of the polarity of the solution and they all fall in the ultraviolet range of 340-380nm. It shows that the fluorescent whitening agent can absorb the ultraviolet light falling in the interval.
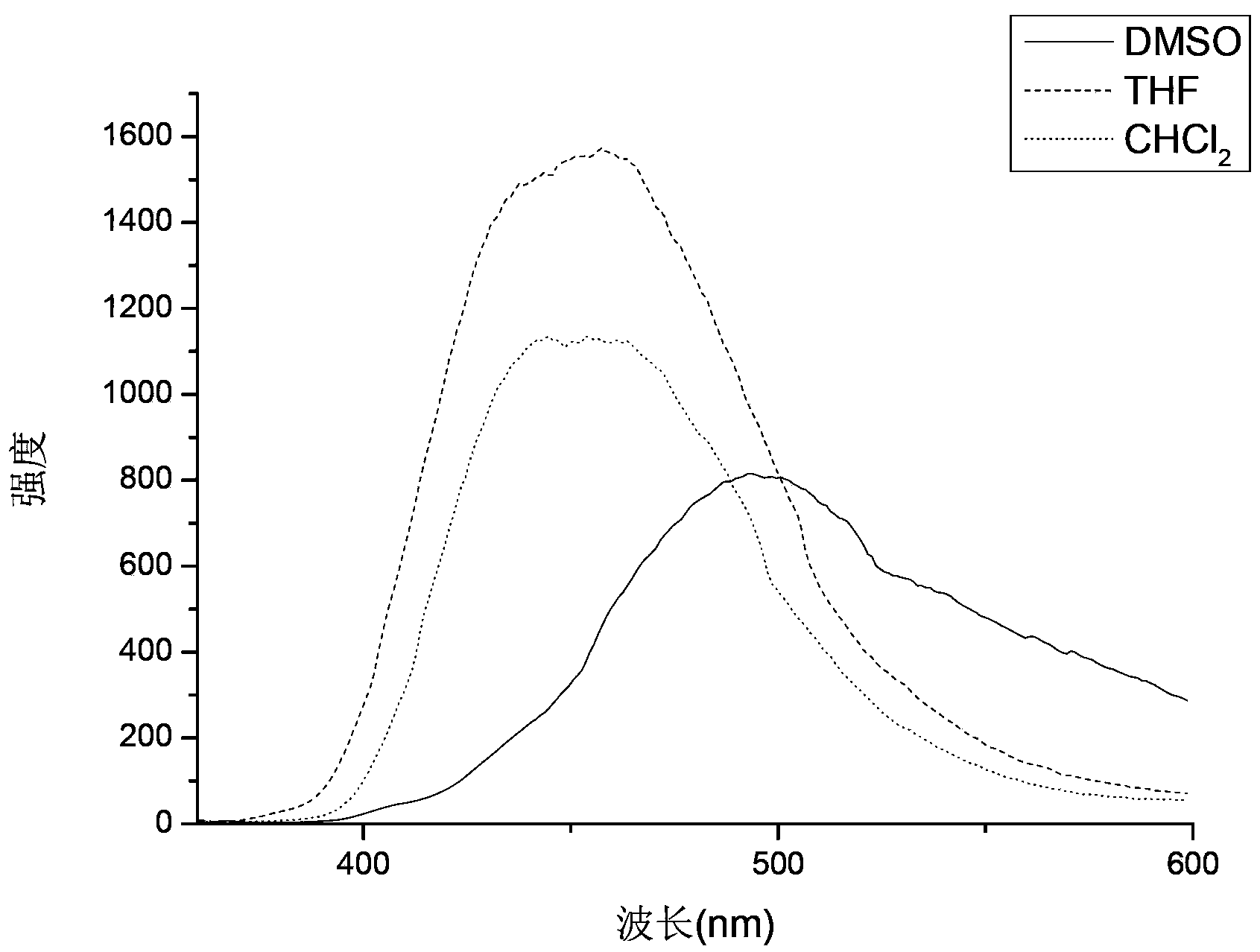
[0036] 2. Determination of fluorescence intensity of 1,3,4-oxadiazole stilbene fluorescent whitening agent
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com