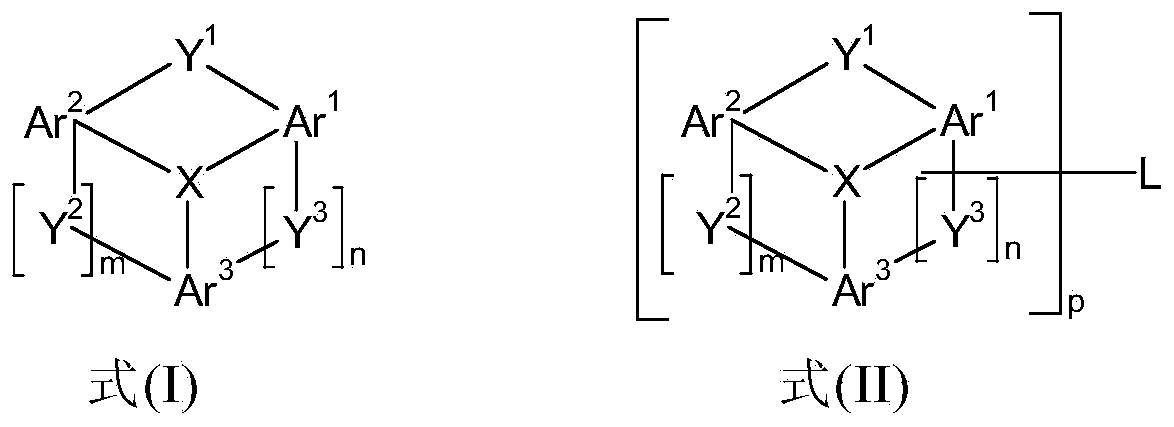
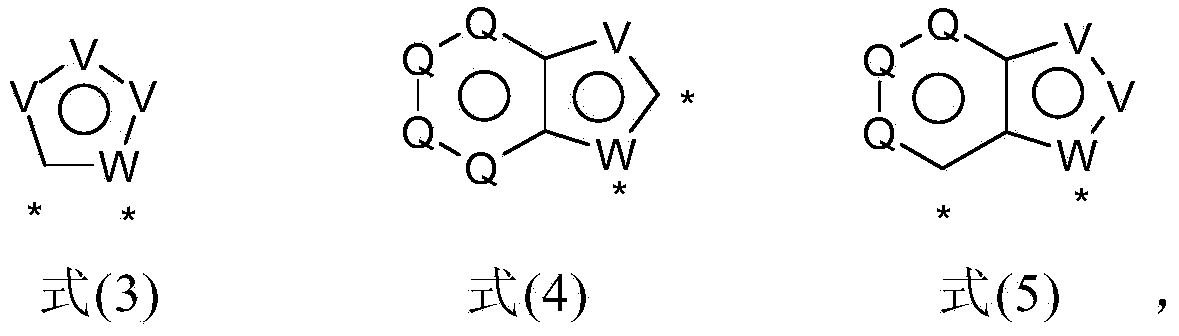
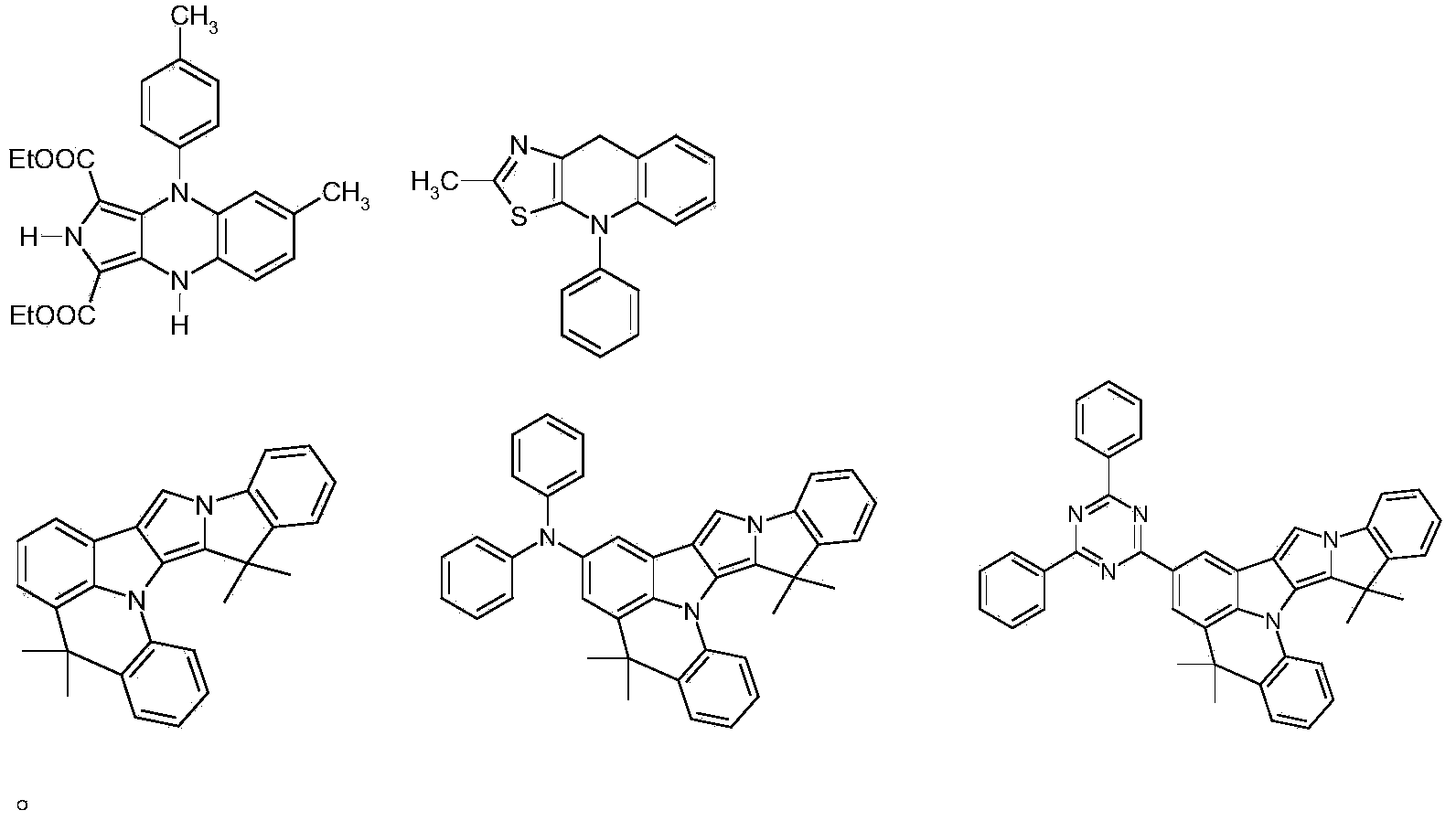
Materials for organic electroluminescent devices
A technology of conditions and groups, applied in the field of materials used in organic electroluminescent devices, can solve problems such as not disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0184] Example 1a: 10-Bromo-8,8-dimethyl-8H-9-thio-11b-azacyclo[a]fluoranthene (Compound 1a)
[0185]
[0186] Step 1: Methyl 3-carbazol-9-ylthiophene-2-carboxylate
[0187] Under protective gas, 102g (420mmol) 3-phenyl-9H-carbazole, 92g (420mmol) methyl 3-bromothiophene-2-carboxylate, 24g (375mmol) copper powder, 104g (757mmol) potassium carbonate and 11g (42mmol) of 18-crown-6 was first introduced into 1200ml of DMF and heated at 130°C for 86 hours. The mixture was then evaporated, washed by stirring with hot heptane, and purified by chromatography (heptane:dichloromethane 1:1). The product was washed by stirring with hot hexanes and a solid was isolated. Yield: 121 g (397 mmol), 65% of theory, according to 1 The purity by H NMR is about 97%.
[0188] Step 2: 2-(3-carbazol-9-ylthiophen-2-yl)propan-2-ol
[0189] 85 g (277 mmol) of methyl 3-carbazol-9-ylthiophene-2-carboxylate were dissolved in 1700 ml of dry THF and degassed. The mixture was cooled to -78°C and 740 m...
Embodiment 2a
[0198] Example 2a: 3-Bromo-8,8-dimethyl-8H-9-thia-11b-azacyclo[a]fluoranthene (Compound 2a)
[0199]
[0200] Step 1: Methyl 3-(3-bromocarbazol-9-yl)thiophene-2-carboxylate
[0201] Cool 63.5g (207mmol) of 8,8-dimethyl-8H-9-thia-11b-azacyclopenta[a]fluoranthene in 2L DMF to -10°C, and add 37.3 g (207 mmol) NBS. The mixture was then allowed to reach room temperature and stirred at this temperature for 6 hours. 500ml of water was then added to the mixture, followed by CH 2 Cl 2 extract. The organic phase was washed with MgSO 4 Dry and remove solvent in vacuo. The solid was isolated by washing the product with stirring with hot toluene. Yield: 72 g (186 mmol), 90% of theory, according to 1 The purity by H NMR is about 97%.
[0202] Step 2: 2-[3-(3-Bromocarbazol-9-yl)thiophen-2-yl]propan-2-ol
[0203] 106 g (277 mmol) of methyl 3-(3-bromocarbazol-9-yl)thiophene-2-carboxylate were dissolved in 1700 ml of dry THF and degassed. The mixture was cooled to -78°C and 740 ml...
Embodiment 3a
[0210] Example 3a: 8,8-Dimethyl-8H-9-thia-11b-azo[a]fluoranthene-10-boronic acid (Compound 3a)
[0211]
[0212] Dissolve 85 g (233 mmol) of 10-bromo-8,8-dimethyl-8H-9-thia-11b-azacyclopenta[a]fluoranthene in 1400 ml of dry THF at -70 °C 121 ml (303 mmol) of a 2.5 M solution of n-butyllithium in cyclohexane were added dropwise, and after 1 hour 33 ml of trimethylborate (302 mmol) were added dropwise, the mixture was allowed to reach room temperature over the course of 1 hour, Removing the solvent, will be based on 1 H-NMR showed a homogeneous residue which was used in subsequent reactions without further purification. The yield is 69 g (207 mmol), corresponding to 90% of theory.
[0213] Compounds 3b-3o were obtained analogously:
[0214]
[0215]
[0216]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com