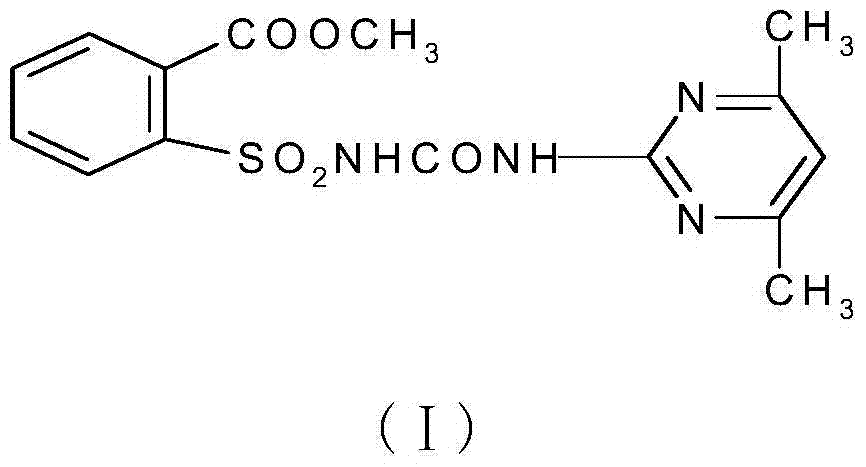Synthetic method of 2-(4,6-dimethylpyrimidine-2-ylamidoformamidosulfonyl)methyl benzoate
A technology of methyl carbamoylsulfamoyl and o-methoxycarbonylbenzenesulfonyl formate, applied in the field of synthesis of methyl 2-benzoate, can solve severe corrosion, high equipment requirements, large pollution and corrosion, etc. problems, to achieve the effects of avoiding safety accidents, good purity, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
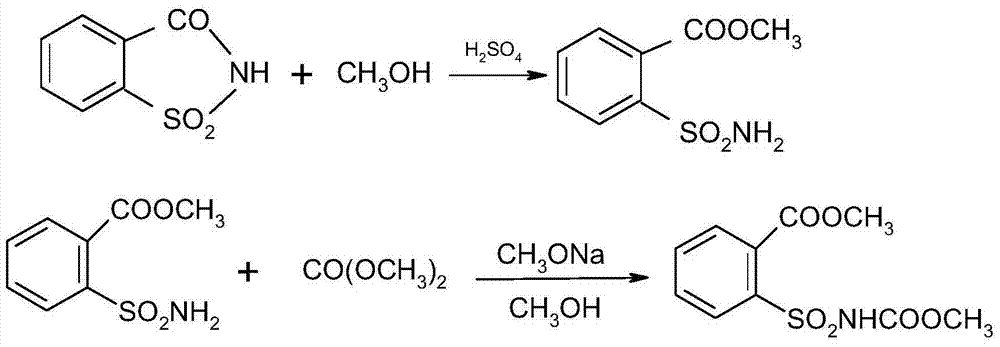
[0039] 1) First, add o-benzoylsulfonimide (saccharin) (20g, 0.11mol), 70mL of methanol, and 98% concentrated sulfuric acid (2g, 0.02mol) into a 250mL three-necked flask, heat and reflux for 3 hours, and then Concentrate under reduced pressure to remove methanol, wash with water until neutral, filter, and dry to obtain 21.7 g of intermediate o-methoxycarbonylbenzenesulfonamide with a content of 98% and a yield of 90%. mp: 122-124°C.
[0040] IR (cm -1 ): 3338, 3235, 3093, 2953, 1716, 1590, 1600, 1557, 1337, 1270, 1161.
[0041] 1 H NMR (δ ppm): 3.84 (3H, s), 7.30 (2H, s), 7.70 (3H, m), 7.98 (1H, d).
[0042] 2) Raise the temperature to 70°C-75°C, under the protection of nitrogen, add o-methoxycarbonylbenzenesulfonamide (21.5g, 0.1mol) and sodium methoxide (21.6g, 0.12mol) into the three-necked flask, and then add carbonic acid dropwise Dimethyl ester (10.8g, 0.12mol) was refluxed for 8 hours, then the solvent was distilled off, the temperature was lowered to below 40°C, and...
Embodiment 2
[0050] 1) Add saccharin (20g, 0.11mol), 30mL of methanol, and concentrated sulfuric acid (2.0g, 0.02mol) into a 250mL three-neck flask, heat to reflux for 3 hours, cool to below 10°C, and slowly add sodium methoxide solution (26.0g, 0.143mol), heat up to 70℃~75℃ for 2h, then add dimethyl carbonate (10.8g, 0.12mol) dropwise within 1h, then reflux for 8h, then distill off the solvent, add 50ml of water after cooling down, and finally use mass The hydrochloric acid with a concentration of 5% was neutralized to neutral, filtered and dried to obtain 24.8 g of methyl o-methoxycarbonylbenzenesulfonamide formate, the content was 98%, and the yield was 81%.
[0051] 2) After adding 100ml of DMF, methyl o-methoxycarbonylbenzenesulfonamide formate (13.7g, 0.05mol) and 4,6-dimethylpyrimidinamine (6.15g, 0.05mol) into a three-neck flask, heat to 100°C-105°C, react for 16h; evaporate the solvent under reduced pressure, cool to room temperature, add water to the bottle, filter, wash with wat...
Embodiment 3
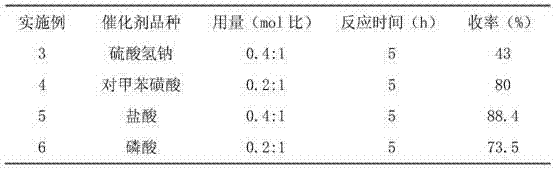
[0054] The difference between this example and Example 1 is that the catalyst A used in the step (1) is sodium bisulfate. The corresponding yields are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com