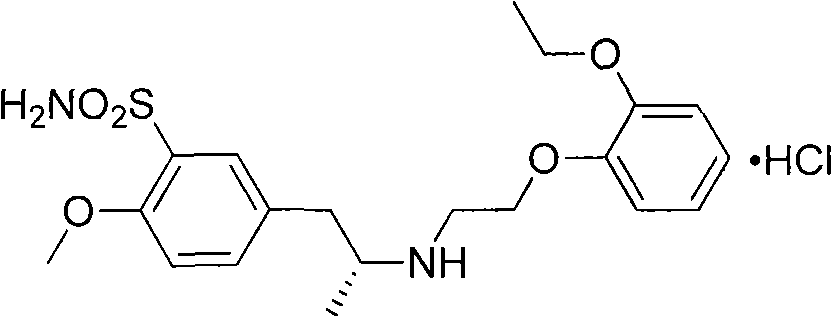
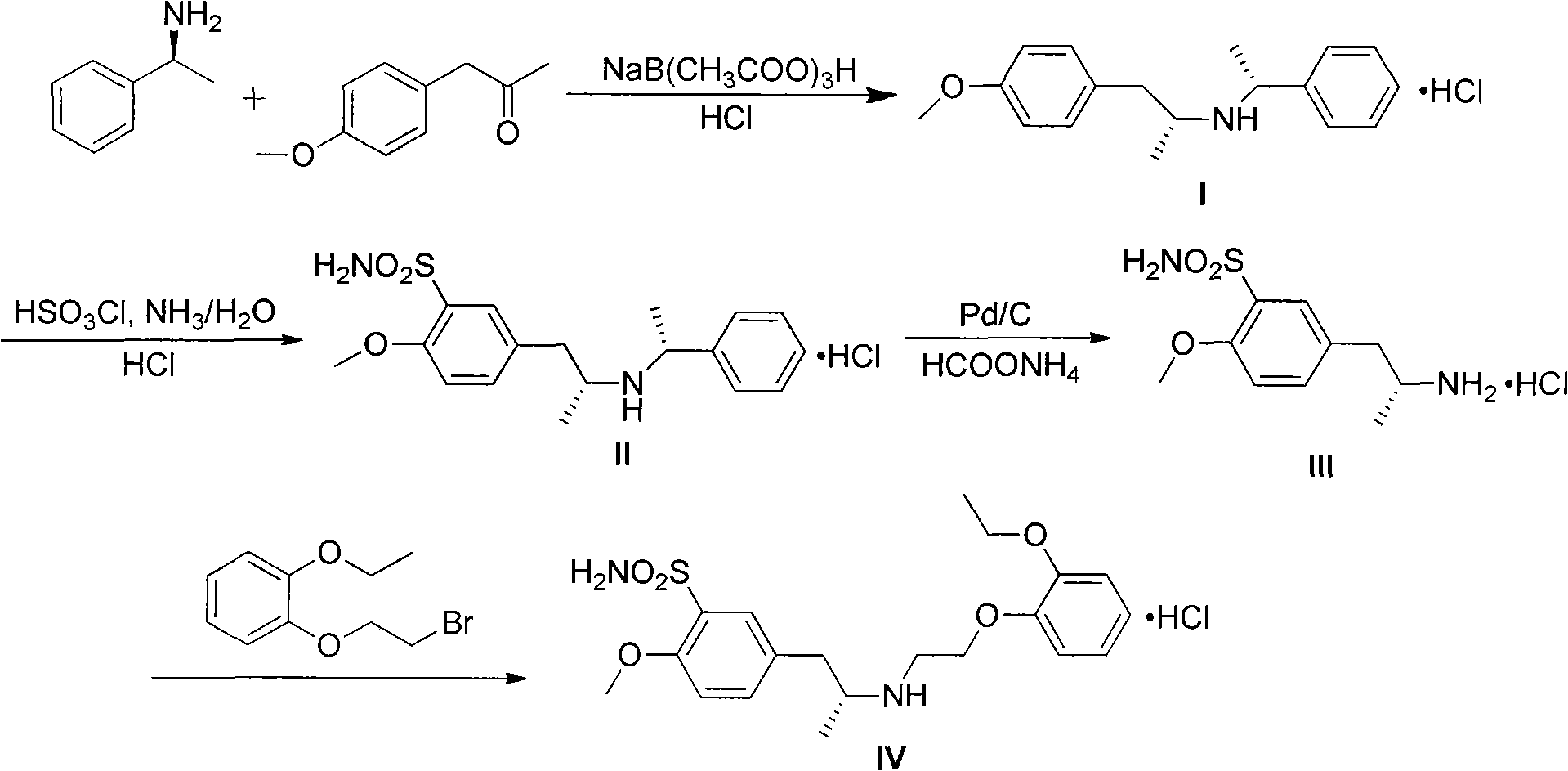
Preparation method for tamsulosin hydrochloride
A technology of tamsulosin hydrochloride and compound, applied in the field of preparation of tamsulosin hydrochloride, can solve problems such as high price and unfriendly environment, and achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Preparation of sodium triacetoxyborohydride
[0020] Add 1000mL of toluene into a 10L three-necked reaction flask, and install mechanical stirring. Stirring was started, 38g of sodium borohydride was added, and an ice-water bath was added to the outside to cool down. 183 g of glacial acetic acid was added dropwise, and the mixture was stirred and reacted overnight. Filter, wash the filter cake with about 200 mL of toluene, filter dry, and place the obtained white solid in a vacuum oven to dry in vacuo to obtain the product for later use.
Embodiment 2
[0021] Example 2: Preparation of N-[(1R)-2-(4-methoxyphenyl)-1-methylethyl]-N-[(1R)-1-phenylethyl]amine hydrochloride
[0022] Put 1300mL of dichloromethane, 93.2g of R-α-phenylethylamine and 114.8g of p-methoxyphenylacetone into a 2000mL three-necked flask equipped with mechanical stirring, start stirring and add an ice-water bath to cool down, then add 42.0g of glacial acetic acid. Add 178.0 g of sodium triacetoxyborohydride in batches, after the addition is complete, stir the reaction at 10°C to 20°C, and track the reaction until complete by thin-layer chromatography. Add 700mL sodium hydroxide aqueous solution (containing about 56g of sodium hydroxide) to adjust the pH value to 9, separate the dichloromethane layer, then add 500mL hydrochloric acid aqueous solution to adjust the pH value to 1-2, separate the dichloromethane layer, add anhydrous Na2SO4 dried. The dichloromethane was recovered by distillation under reduced pressure to dryness, 1500 mL of acetone was added t...
Embodiment 3
[0023] Example 3: Preparation of 5-[[(2R)-2-[(1R)-N-(1-methylbenzyl)]amino]propyl]-2-methoxybenzenesulfonamide hydrochloride
[0024] Add 168mL of chlorosulfonic acid and 700mL of dichloromethane into a 1000mL three-necked flask, install mechanical stirring, add dropwise at room temperature the solution prepared by dissolving 128g of the reaction product from the previous step in 100mL of dichloromethane, dropwise, 0℃~10℃ The reaction was stirred overnight. Under stirring, the reactant was added dropwise into a reaction flask filled with 1600mL of ammonia water, 2000mL of ethyl acetate and 1000g of ice, and the temperature was kept at -5°C to 5°C for cooling in an external ice-salt bath. After dropping, the reaction was stirred for about 4 hours, and the reaction was followed by thin-layer chromatography to complete. Separate the organic layer, wash with sodium chloride aqueous solution 4 times, 800 mL each time, separate the organic layer, add anhydrous sodium sulfate to dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com