Verapamil hydrochloride sustained release microsphere and preparation method thereof
A technology of verapamil hydrochloride and sustained-release microspheres, which is applied in the directions of pharmaceutical formulations, medical preparations of inactive ingredients, and pill delivery, etc. Unique, increase the ability to control drug release, improve drug distribution uniformity and particle size uniformity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
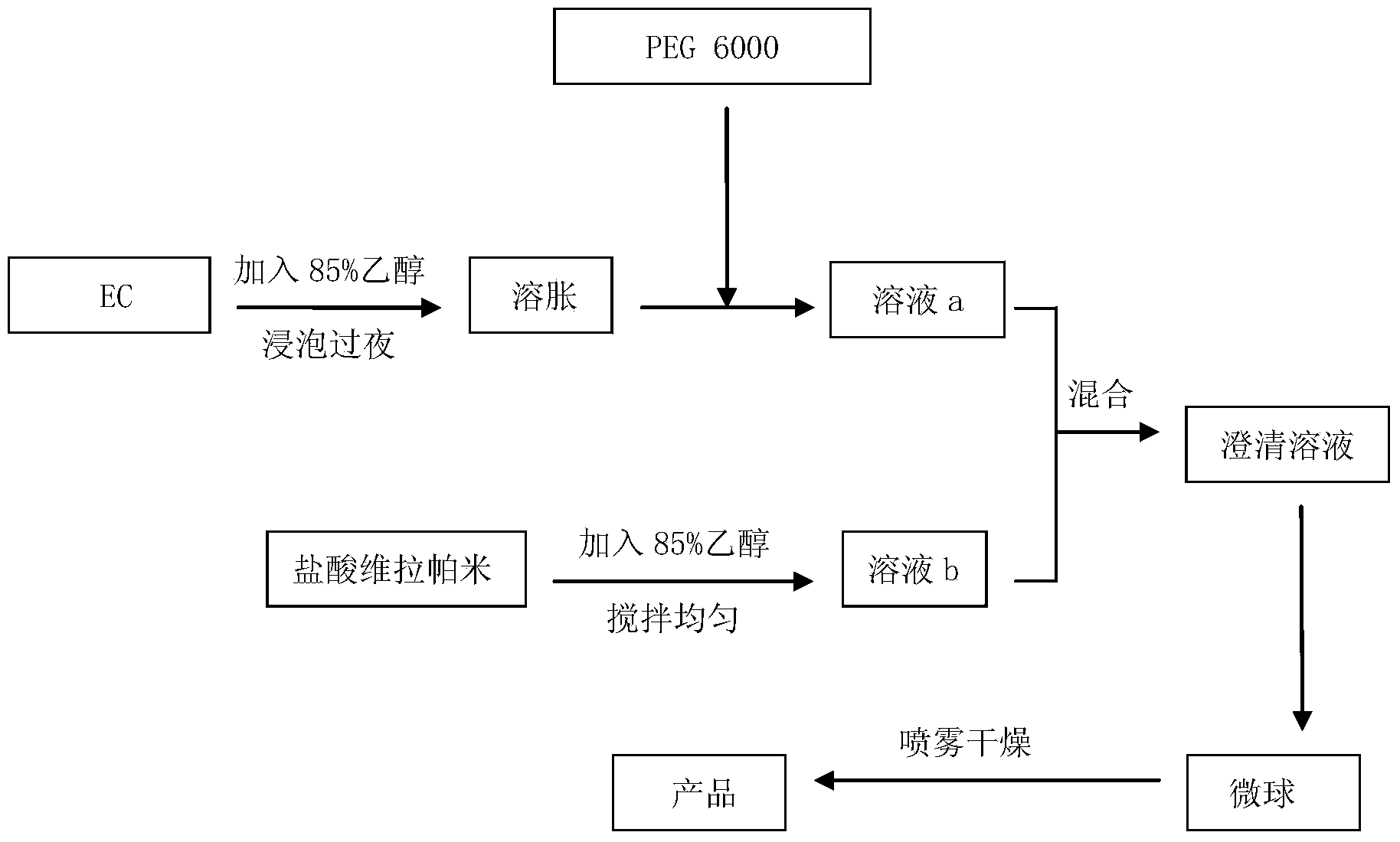
[0027] (1) Take ethyl cellulose (EC) with a viscosity of 7CP, add 85% ethanol to make the mass concentration 1:10, let it stand and soak overnight to fully swell, and then stir to dissolve.
[0028] (2) Add PEG400 to the above solution at 25°C, stir evenly, and emulsify for 10 minutes to obtain solution a.
[0029] (3) Weigh EC and verapamil hydrochloride with a mass ratio of 2:1, and add verapamil hydrochloride (crushed through a 300 mesh sieve) with 85% ethanol solution with a mass concentration of 1:5, and stir well to obtain Solution b.
[0030] (4) Add solution b to solution a under the stirring state of 250rpm to form an emulsified system. Keep the stirring state and stir completely for 30 minutes. The outlet air temperature of the spray dryer is 40°C, and the inlet air temperature is 75°C It can be obtained after spray drying.
Embodiment 2
[0032] (1) Take ethyl cellulose (EC) with a viscosity of 50CP, add 85% ethanol to make the mass concentration 1:20, let it stand and soak overnight to fully swell, and then stir to dissolve.
[0033] (2) Add PEG600 to the above solution at 40°C, stir evenly, and emulsify for 20 minutes to obtain solution a.
[0034] (3) Weigh the mass ratio of EC and verapamil hydrochloride to be 3.5:1, and add verapamil hydrochloride (crushed through a 300 mesh sieve) with 85% ethanol solution with a mass concentration of 1:15, and stir well to obtain Solution b.
[0035](4) Add solution b to solution a under the stirring state of 300rpm to form an emulsified system. Keep the stirring state and stir completely for 80 minutes. The outlet air temperature of the spray dryer is 55°C, and the inlet air temperature is 90°C It can be obtained after spray drying.
Embodiment 3
[0037] (1) Take ethyl cellulose (EC) with a viscosity of 100CP, add 85% ethanol to make the mass concentration 1:30, let it stand and soak overnight to fully swell, and then stir to dissolve.
[0038] (2) Add polyethylene glycol PEG8000 to the above solution at 50°C, stir evenly, and emulsify for 30 minutes to obtain solution a.
[0039] (3) Weigh EC and verapamil hydrochloride with a mass ratio of 5:1, and add verapamil hydrochloride (crushed through a 300 mesh sieve) with 85% ethanol solution with a mass concentration of 1:25, and stir well to obtain Solution b.
[0040] (4) Add solution b to solution a under the stirring state of 400rpm to form an emulsified system. Keep the stirring state and stir completely for 120 minutes. The outlet air temperature of the spray dryer is 70°C, and the inlet air temperature is 105°C It can be obtained after spray drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com