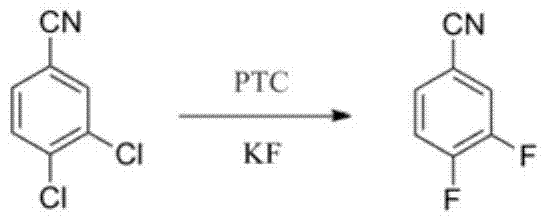
A kind of synthesis technique of 3,4-difluorobenzonitrile
A technology of difluorobenzonitrile and synthesis process, applied in 3, can solve the problems of complicated post-treatment, serious dehalogenation side reaction, long reaction time, etc., and achieve the effects of good thermal stability, less discharge of three wastes, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 150g of 3,4-dichlorobenzonitrile, 450g of 1,3-dimethyl-2-imidazolidinone, 100g of cyclohexane into a 1000ml reactor with stirrer, reflux separator and thermometer, and heat up to 90°C. Reflux water diversion 1 hour, after treating that there is no water in the water separator, drop into 75g spray-dried potassium fluoride, two-(N-bis(dimethylamino)methylene)-imine chloride 10g, be warming up to 140°C, reflux and water separation for 3 hours to obtain the intermediate 3-chloro-4fluorobenzonitrile, continue to heat up to 180°C, and the reaction is over for 5h.
[0030] Dilute the obtained reaction solution with toluene and filter under reduced pressure to remove salt, wash the filter cake 3 times with toluene, transfer the filtrate to a rectification kettle for rectification under reduced pressure, control the vacuum degree to 0.08-0.09MPa, and collect The 105°C fraction was 79g, the GC purity was 99%, and the yield was 65%.
Embodiment 2
[0032] Add 150g of 3,4-dichlorobenzonitrile, 450g of 1,3-dimethyl-2-imidazolidinone, 100g of toluene into a 1000ml reaction kettle equipped with a stirrer, reflux separator, and thermometer, heat up to 120°C, and reflux Water for 2 hours, after no water comes out in the water separator, put in 150g of spray-dried potassium fluoride, 15g of bis-(N-bis(dimethylamino)methylene)-imide chloride, and heat up to 130°C , reflux and water separation reaction for 2 hours to obtain the intermediate 3-chloro-4fluorobenzonitrile, continue to heat up to 200 ° C, and the reaction is completed for 6 hours.
[0033] Dilute the obtained reaction liquid with toluene, then filter under reduced pressure, remove salt, wash the filter cake with toluene 3 times, transfer the filtrate to a rectification kettle for rectification under reduced pressure, control the vacuum degree to 0.08-0.09MPa, and collect at the top of the tower 84g fraction at 90-105°C, GC purity 99%, yield 70%.
Embodiment 3
[0035] Add 150g of 3,4-dichlorobenzonitrile, 300g of 1,3-dimethyl-2-imidazolidinone, 100g of toluene into a 1000ml reactor with stirrer, reflux separator, and thermometer, heat up to 120°C, reflux Water for 1.5 hours, after no water has come out in the water separator, put in 150g of spray-dried potassium fluoride, 15g of bis-(N-bis(dimethylamino)methylene)-imide chloride, and heat up to 150°C , refluxed and separated water for 2.5 hours to obtain the intermediate 3-chloro-4fluorobenzonitrile, and continued to heat up to 180°C, and the reaction was completed for 5 hours.
[0036] Dilute the obtained reaction liquid with toluene, then filter under reduced pressure, remove salt, wash the filter cake with toluene 3 times, transfer the filtrate to a rectification kettle for rectification under reduced pressure, control the vacuum degree to 0.08-0.09MPa, and collect at the top of the tower The fraction at 90-105°C was 87.3g, the GC purity was 99%, and the yield was 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com