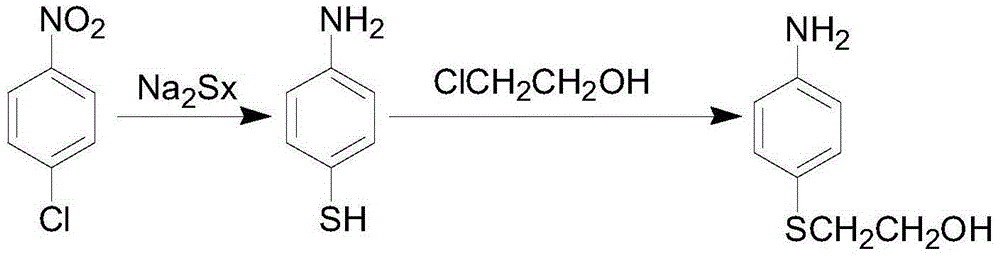
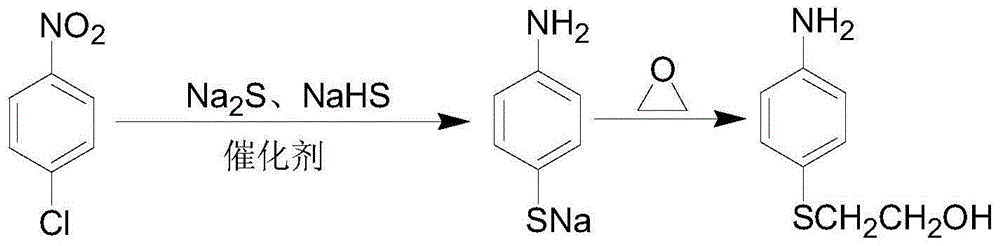
The preparation method of p-aminophenyl-β-hydroxyethyl sulfide
A technology of hydroxyethyl sulfide and p-aminophenyl, which is applied in the field of dye intermediate preparation, can solve the problems of weak alkylation ability of 2-chloroethanol, high price of 2-chloroethanol, complicated and uncontrollable operation process, etc. Achieve the effects of suppressing side reactions, simple and easy preparation process, and simple and easy-to-control operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 240mL of water into a 500mL four-neck flask, add 95.8g of sodium sulfide (industrial product) and 59.5g of sodium hydrosulfide (industrial product) under stirring, and raise the temperature to 40-60°C. Continue to stir until the sodium sulfide and sodium hydrosulfide are completely dissolved, add 0.8 g of activated carbon, stir for half an hour to decolorize, and collect the filtrate by suction filtration for later use.
[0030] Add 200mL of water, 100g of p-nitrochlorobenzene, and 0.6g of N-methylpyrrolidone into a 1000mL four-neck flask, stir and heat to 92-96°C, then add the above-mentioned filtrate dropwise at this temperature, and drop it in about 1.5 hours. Finally, continue to stir and keep warm for 7 hours until the end of the reaction. After the reaction is over (the reaction solution is tested by HPLC: the HPLC content of p-aminothiophenate sodium is 90.5%, and the HPLC content of the by-product p-chloroaniline is 2.5%), the reaction device is changed to a...
Embodiment 2
[0033]Add 240mL of water into a 500mL four-necked flask, add 114.4g of sodium sulfide (industrial product) and 73.2g of sodium hydrosulfide (industrial product) under stirring, and raise the temperature to 40-60°C. Continue to stir until the sodium sulfide and sodium hydrosulfide are completely dissolved, add 0.8 g of activated carbon, stir for half an hour to decolorize, and collect the filtrate by suction filtration for later use.
[0034] Add 200mL of water, 100g of p-nitrochlorobenzene, and 0.9g of N-ethylpyrrolidone into a 1000mL four-neck flask, stir and heat to 100-105°C, then add the above-mentioned filtrate dropwise at this temperature, and drop it in about 1.5 hours. Finally, continue to stir and keep warm for 6 hours until the end of the reaction. After the reaction is over (the reaction solution is tested by HPLC: the HPLC content of p-aminothiophenate sodium is 91.0%, and the HPLC content of the by-product p-chloroaniline is 2%), the reaction device is changed to ...
Embodiment 3
[0037] Add 240mL of water into a 500mL four-necked flask, add 104g of sodium sulfide (industrial product) and 66g of sodium hydrosulfide (industrial product) under stirring, and raise the temperature to 40-60°C. Continue to stir until the sodium sulfide and sodium hydrosulfide are completely dissolved, add 0.8 g of activated carbon, stir for half an hour to decolorize, and collect the filtrate by suction filtration for later use.
[0038] Add 200mL of water, 100g of p-nitrochlorobenzene, and 1g of N-methylpyrrolidone into a 1000mL four-neck flask, stir and heat to 95-98°C, then add the above-mentioned filtrate dropwise at this temperature for about half an hour, and finally Continue to stir and keep warm for 8 hours until the end of the reaction. After the reaction (the reaction liquid is tested by HPLC: the HPLC content of p-aminothiophenate sodium is 92%, and the HPLC content of the by-product p-chloroaniline is 3%), the reaction device is changed to a distillation device, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com