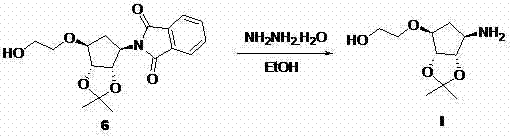Method for preparing ticagrelor key intermediate
A technology for ticagrelor and intermediates, which is applied in the new synthesis field of the key intermediates of the anticoagulant drug ticagrelor, can solve the problems of complex post-processing, high risk, and high energy consumption of reduction reaction, and achieve environmental protection Friendly, mild reaction conditions, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 [3aS-(3aα,4α,6α,6aα)]-[2,2-Dimethyl-6-(hydroxyethyl)-tetrahydro-4H-cyclopenta-1,3-dioxolane Preparation of -4-yl-carbamic acid-benzyl methyl ester
[0035]
[0036] Add compound 2 (1 g, 3.25 mmol, 1 eq.) and anhydrous tetrahydrofuran (20 mL) into a dry three-necked flask. Cool to 0 °C, add potassium tert-butoxide (0.73 g, 6.51 mmol, 2 eq.) under nitrogen protection, and stir at this temperature for 2 hours, then add 1,3,2-dioxazolethiophene-2,2 - Dioxide (IIIa), stirred at room temperature for 1 hour. After the reaction was complete, concentrated sulfuric acid (2 mL) was added to the reaction solution, stirred for 5 minutes, and intermediate 4 was completely reacted. The reaction solution was concentrated to dryness to remove the solvent, and recrystallized from absolute ethanol to obtain 3 as a white solid. Yield: 0.45 g, Yield: 39%.
[0037] 1 HNMR (400MHz, CDCl 3 ), δ7.31(m,5H), 5.74(d,1H), 5.11(d,2H), 4.55(m,2H), 4.17(m,1H), 3.88(m,1H), 3.72(m, ...
Embodiment 2
[0038] Example 2 [3aR-(3aα, 4α, 6α, 6aα)]-2-[[6-amino-2,2-dimethyl-tetrahydro-4H-cyclopenta-1,3-dioxolane- Preparation of 4-yl]oxo]-ethanol
[0039]
[0040] Compound 3 (4g, 5.7mmol) was dissolved in absolute ethanol (20mL), and a catalytic amount of 10% palladium carbon was added, and stirred overnight under a hydrogen atmosphere. After the reaction was detected by TLC, it was filtered, and the filter cake was washed three times with anhydrous methanol. The filtrate was concentrated to obtain compound 10 as a light yellow oil (TLC: DCM : MeOH = 8:1). Yield: 2.2 g, Yield: 93%.
[0041] 1 H NMR (400 MHz, CDCl 3 ) δ 4.57 (d, 1H), 4.37 (d, 1H), 3.79 (d, 1H), 3.61 – 3.46 (m, 4H), 3.26 (d, 1H), 3.02 (s, 3H), 2.10 - 2.01 ( m, 1H), 1.73 (d, 1H), 1.33 (s, 3H), 1.20 (s, 3H).
Embodiment 3
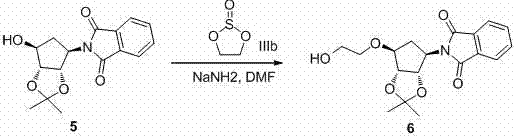
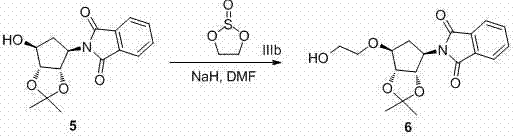
[0042] Example 3 2-[(3aS,4R,6S,6aR)-6-hydroxy-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxolane-4- Preparation of alcohol]isoindoline-1,3-dione
[0043]
[0044] Add 1 (3 g, 17.3 mmol, 1 eq.), phthalic anhydride (2.6 g, 17.3 mmol, 1 eq.) and DIPEA (3.7 mL, 22.5 mmol, 1.3 eq.) in a stuffy jar, and Sealed reaction at ℃ for 5 hours. Cool to room temperature, add dichloromethane and water to the reaction liquid, separate layers, collect the organic phase, dry and filter, concentrate to obtain the crude product, and column chromatography obtains 2 g of solid, yield: 40%.
[0045] 1 HNMR (400MHz, CDCl 3 ):7.89(m, 2H), 7.78(m, 2H), 5.11(m, 1H), 4.73(m, 2H), 4.32(m, 1H), 3.75(d, 1H), 2.67(m, 1H) , 2.01(m, 1H), 1.52(s, 3H), 1.27(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com