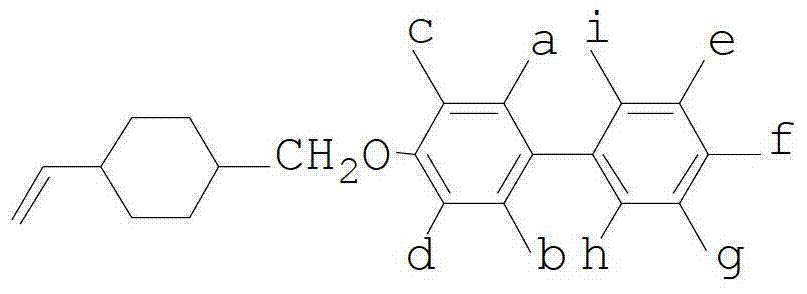
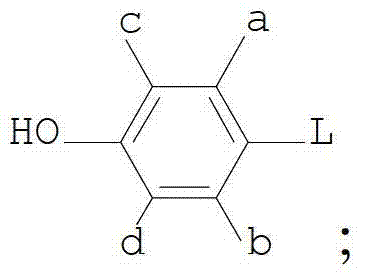
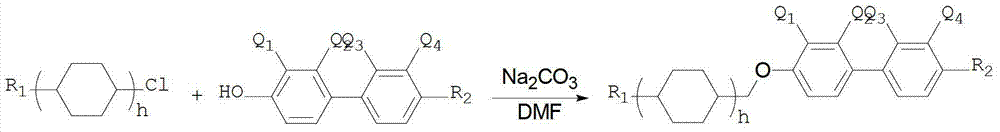Vinyl cyclohexyl methyl ether liquid crystal compounds and preparation method thereof
A technology of liquid crystal compound and hexyl methyl ether, which is applied in the field of fine chemical industry and material chemistry, can solve the problems of insufficient liquid crystal range and no compound involved, and achieve the effects of lower threshold voltage, large dielectric anisotropy, and lower power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] 2. Preparation of methyl 4-formylcyclohexylcarboxylate
[0083]
[0084] Experimental device: 1 liter three-necked flask, equipped with thermometer, stirring, N 2 Protection, ice bath.
[0085] Operation process: Add 56.8g of the above product and 200mL of THF to a three-necked flask, add 500mL of 5% hydrochloric acid dropwise, and control the temperature to be less than 10°C. After the addition is complete, heat to 30°C and keep it for 4 hours. Add 200mL water, use 200mL CH 2 Cl 2 Extract, wash with water to neutral, add anhydrous Na 2 SO 4 After drying, the solvent was removed with a rotary evaporator to obtain 48.5 g of light yellow liquid, yield: 96.0%, GC purity: 90.2%.
[0086] 3. Preparation of methyl 4-vinylcyclohexylcarboxylate
[0087]
[0088] Experimental device: 2-liter three-necked flask, equipped with thermometer, stirring, liquid sealing, N 2 Protection, drying tube.
[0089] Operation process: add CH to the three-necked bottle 3 PPh 3 Br106.8g and 200mLTHF. Pu...
Embodiment 1
[0102] Example 1: Preparation of VC10PUF
[0103] 1. Preparation of OHPUF
[0104]
[0105] Method a: Experimental device: 500mL three-necked flask, liquid seal, mechanical stirring, 0-100°C thermometer, reflux condenser, drying tube.
[0106] Operation process: Add 27.6g potassium carbonate and 55g water into a three-necked flask under the protection of nitrogen, stir and dissolve completely, add 17.6g 3,4,5-trifluorophenylboronic acid, 17.3g p-iodophenol, 0.224g tetratriphenylphosphine Palladium and 80g toluene were incubated at 70~90℃ for 3h. After the reaction, 200mL of water was added to the system and transferred to a separatory funnel. The organic phase was washed with water to neutrality, and the solvent was removed by a rotary evaporator to obtain 26g of crude product. Toluene and n-hexane were recrystallized once to obtain 19.6 g of product, with a yield of 87.0% and a GC purity of 98.2%.
[0107] Method b: Experimental device: 500mL three-necked flask, liquid seal, mechan...
Embodiment 2
[0119] Example 2: Preparation of VC10UUF
[0120] 1. Preparation of VC10U
[0121]
[0122] Method a: Experimental device: 500mL three-necked flask, liquid seal, mechanical stirring, 0~300℃ thermometer, reflux condenser, drying tube.
[0123] Operation process: Add 50g VCMI, 27.3g 3,5-difluorophenol, 22.4g potassium hydroxide and 200g toluene to a three-necked flask under the protection of nitrogen. The temperature is raised to reflux, and the reaction is kept for 10 hours. After the reaction, it is cooled to room temperature and added to the system. Add 200 mL of water, transfer to a separatory funnel, wash the organic phase with water to neutrality, remove the solvent on the rotary evaporator to obtain 48 g of the product, and recrystallize it twice with toluene and absolute ethanol to obtain 43.7 g of the product, yield 87.0%, GC purity 96.4%.
[0124] Method b: Experimental device: 500mL three-necked flask, liquid seal, mechanical stirring, 0~300℃ thermometer, reflux condenser, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com