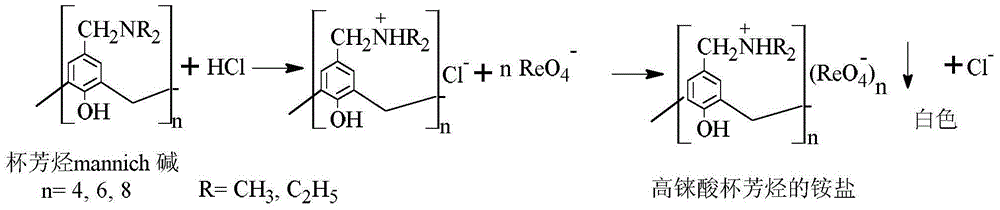Process for Separating and Recovering Rhenium
A technology of perrhenic acid and calixarene, applied in chemical instruments and methods, rhenium compounds, inorganic chemistry, etc., can solve the problems of poor anti-interference ability, complicated process, low adsorption capacity, etc., and achieve cost saving, high purity, The effect of high extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] (1) Preparation of sample solution containing rhenium: Re(VII) standard solution: Accurately weigh 1.0000 g of rhenium powder dried at 105°C to 110°C for 2 hours in a beaker, wet it with water, add 10mL of hydrogen peroxide, and heat to dissolve Completely, after cooling, add 30mL of hydrochloric acid, shake well, slightly boil for 20min to remove excess hydrogen peroxide, then pour into a 100mL volumetric flask and dilute to the mark to obtain a standard solution of 10mg / mL Re(VII).
[0025] Standard solution of rhenium: 0.01mg·mL -1 , Weigh 0.1000g of rhenium powder, add 10-20mL of nitric acid to a 100ml beaker, place in a heating mantle and heat slowly until completely dissolved. Then add 20ml of hydrochloric acid, heat to drive out NO 3 - Until no brown-yellow gas is emitted. Remove and cool to room temperature. Transfer to a 100ml volumetric flask, add water to dilute to the mark, and shake well. Then take 1ml of the above solution in a 100mL volumetric flask,...
Embodiment 1
[0028] Dissolve 0.4mmol calix[4]arene diethylamine mannich base in 10mL of 1mol / L hydrochloric acid solution, add dropwise to 25mL of the standard solution of Re(VII) under stirring, a white solid is formed, stir for 30min and then filter, spectrophotometrically The content of rhenium in the filtrate was determined by the method.
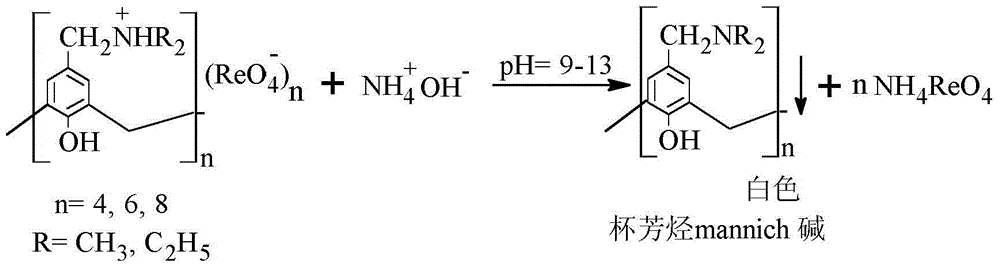
[0029] Add the white solid obtained above to 10 mL of 1mol / L NH 3 ·H 2 In O, keep pH = 10, heat and stir, there is still precipitation in the system, stop stirring and heating after about 30 minutes of reaction, carry out solid-liquid separation, take out 1mL of liquid, measure the concentration of rhenium in the solution according to the above method, and calculate the concentration of rhenium content, the recovery rate is about 85.7%. Then the liquid is further concentrated to finally obtain white crystals of ammonium perrhenate; the solid is filtered, washed and dried to finally obtain calix[4]arene diethylamine mannich base again.
Embodiment 2
[0031] Dissolve 0.4mmol calix[4]arene dimethylamine mannich base in 10mL of 1mol / L hydrochloric acid solution, add dropwise to 25mL of the standard solution of Re(VII) under stirring, a white solid is formed, filter after stirring for 30min, and spectrophotometrically The content of rhenium in the filtrate was determined by the method.
[0032] Add the white solid obtained above to 10 mL of 1mol / L NH 3 ·H 2 In O, keep pH=10, heat and stir, there is still precipitation in the system, stop stirring and heating after reacting for about 30min, carry out solid-liquid separation, take out 1mL of liquid, measure the content of rhenium in the solution according to the above method, the recovery rate is about was 82.4%. Then the liquid is further concentrated to finally obtain white crystals of ammonium perrhenate; the solid is filtered, washed and dried to finally obtain calix[4]arene dimethylamine mannich base again.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com