Preparation method and application of anthraquinone polypyridine ligand and binuclear ruthenium complexes
A technology of anthraquinone polypyridine and anthraquinone dinuclear ruthenium polypyridine, which is applied in the field of preparation of anthraquinone polypyridine ligands and their dinuclear ruthenium complexes, can solve the limitations of clinical application, severe toxicity and drug resistance of platinum complexes In order to achieve the effect of simple synthesis method, inhibition of tumor cell migration and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of Anthraquinone Polypyridine Ligands and Binuclear Ruthenium Complexes
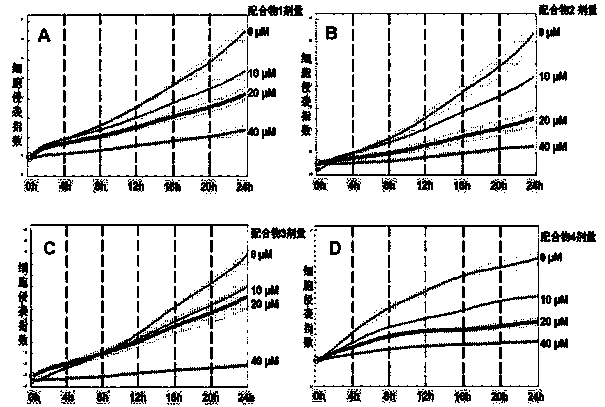
[0032]The general formula of the binuclear ruthenium complex is: [(bpy) 2 Ru (X-biipad)Ru(bpy) 2 ](ClO 4 ) 4 ,in X Anthraquinone polypyridine ligands, specifically: 1,4-bis(imidazo[4,5-f][1,10]phenanthroline-2-)anthracene-9,10-dione (ligand 1); 1,5-bis(imidazo[4,5-f][1,10]phenanthroline-2-)anthracene-9,10-dione (ligand 2); 2,6-bis( imidazo[4,5-f][1,10]phenanthroline-2-)anthracene-9,10-dione (ligand 3); 2,7-bis(imidazo[4,5-f] [1,10]phenanthroline-2-)anthracene-9,10-dione (ligand 4). The binuclear ruthenium complexes prepared from ligands 1-4 correspond to complexes 1-4 respectively.
[0033] (1) Synthesis of 1,4-bis(imidazo[4,5-f][1,10]phenanthroline-2-)anthracene-9,10-dione (1,4-biipad)
[0034] (a) Synthesis of 2-(2',5'-dimethylbenzoyl)benzoic acid
[0035] Can refer to ( Journal of Jiangxi Normal University, 2008, 32 , 1, 94-97) Synthetic method, add 34...
Embodiment 2
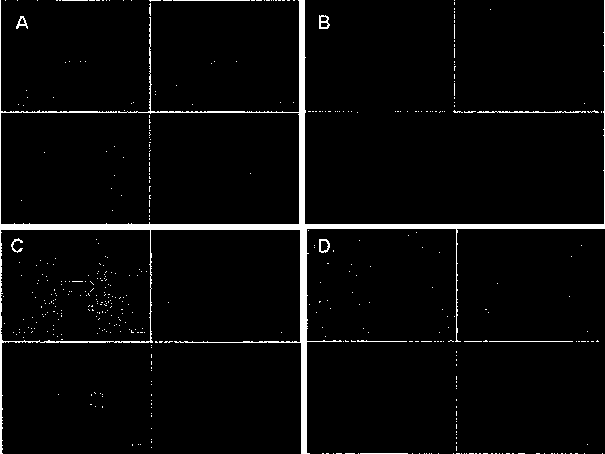
[0076] Example 2 Binuclear ruthenium complexes inhibit the migration of MHCC97H tumor cells
[0077] The principle of cell scratch method: refer to the in vitro cell wound healing experimental model to measure the motility characteristics of tumor cells, scratch and wound the monolayer cells cultured in vitro, and then add drugs to observe its ability to inhibit tumor cell migration. If the drug can inhibit the ability of cell migration, the scratch cannot be covered by the migration movement of the cell, and the degree of healing of the scratch can reflect the strength of the ability of the drug to inhibit cell migration. Experimental steps: set the density to 5 × 10 5 Cells / mL of human hepatoma cell MHCC97H cells were plated on a 24-well plate (0.5 mL / well), added with 10% fetal bovine serum, and cultured for 24 hours to form a monolayer of cells. Then use the tip of a pipette to scratch the monolayer of cells in a straight line, wash with PBS three times, replace with DM...
Embodiment 3
[0078] Example 3 Binuclear Ruthenium Complex Inhibits MHCC97H Tumor Cell Invasion
[0079] Tumor cell invasion test principle: Matrigel is a matrix component extracted from mouse EHS sarcoma, containing LN, type IV collagen, contact protein and heparin sulfate polysaccharide, spread on polycarbonate filter membrane without polyvinylpyrrolidone, can The membrane structure is reconstituted in DMEM medium, which is very similar to the natural matrix structure. The pore size of the filter membrane is generally 8 μm, and the membrane pores are covered by Matrigel. Cells cannot pass through freely. They must secrete hydrolytic enzymes and deform to pass through the filter membrane covered with Matrigel, which is similar to the situation in vivo. The filter membrane covered with Martrigel is placed between the upper and lower chambers of the Blind Well chamber or MICS chamber, and the side covered with Martrigel faces the upper chamber. Chemotactic agents such as a certain concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com