Method for producing N-(phosphonomethyl)iminodiacetic acid and recycling mother solution by hydrogen chloride desalinization
A cyclic application, hydrogen chloride technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of uneconomical process, high energy consumption, and large consumption of vapor to concentrate mother liquor, etc. To achieve the effect of simple and easy method, avoid high energy consumption and avoid cumbersome process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
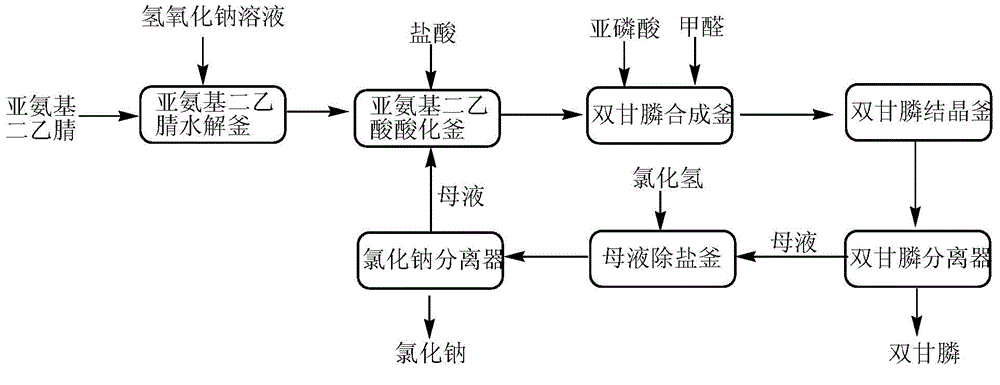
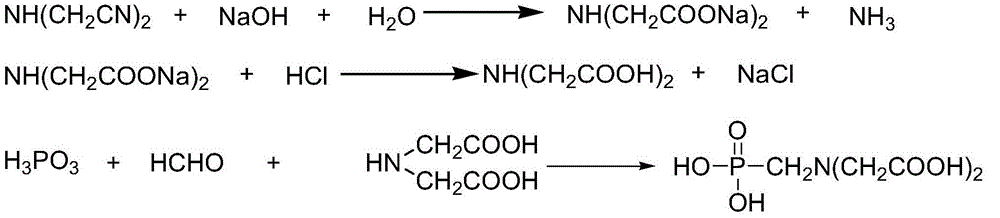
[0024] 1) Add 420g (4.2mol) of 20% sodium hydroxide solution to a stirring four-necked round bottom flask with a thermometer, and then add 200g (95% , 2.0mol), after the addition, keep warm for 1 hour, iminodiacetonitrile is hydrolyzed to obtain iminodiacetic acid disodium salt, and then heated to 90°C to distill water to remove ammonia;
[0025] 2) Add 730g (31%, 6.2mol) of hydrochloric acid to the disodium salt of iminodiacetic acid obtained in step 1) to acidify to obtain a mixture of iminodiacetic acid and sodium chloride;
[0026] 3) Add 192.5g (98%, 2.3mol) of phosphorous acid to the mixture obtained in step 2), heat, distill water (510g of water has been distilled twice), and add 211g of formaldehyde dropwise between 115 and 120°C (content 37%, 2.6mol), heat preservation reaction for 2 hours after the addition, to synthesize diglyphosate;
[0027] 4) Cool the reaction product obtained in step 3) to 10°C, keep it crystallized for 2 hours, filter, wash the filter cake wi...
Embodiment 2
[0030] 1) Prepare a batch of iminodiacetic acid disodium salt under the same conditions as in Example 1;
[0031] 2) Add the separated sodium chloride obtained in step 5) of Example 1 to the disodium salt of iminodiacetic acid and acidify the mother liquor containing hydrogen chloride to obtain a mixture of iminodiacetic acid and sodium chloride;
[0032] 3) Add 171.5g (98%, 2.05mol) of phosphorous acid to the mixture obtained in step 2), heat, distill 180.2g of water, and add dropwise 180.0g of formaldehyde (content 37%, 2.22mol) between 115 and 120°C ), heat preservation reaction for 2 hours after adding, and synthesize diglyphosate;
[0033] 4) Cool the reaction product obtained in step 3) to 10°C, keep it crystallized for 2 hours, filter, wash the filter cake with 30mL of water, and dry to obtain 431.9g of white bisglyphosate product, with a content of 98.3% and a yield of 93.5%; a mother liquor of 523.6 g;
[0034] 5) Concentrate and distill 73g of water from the mother...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com