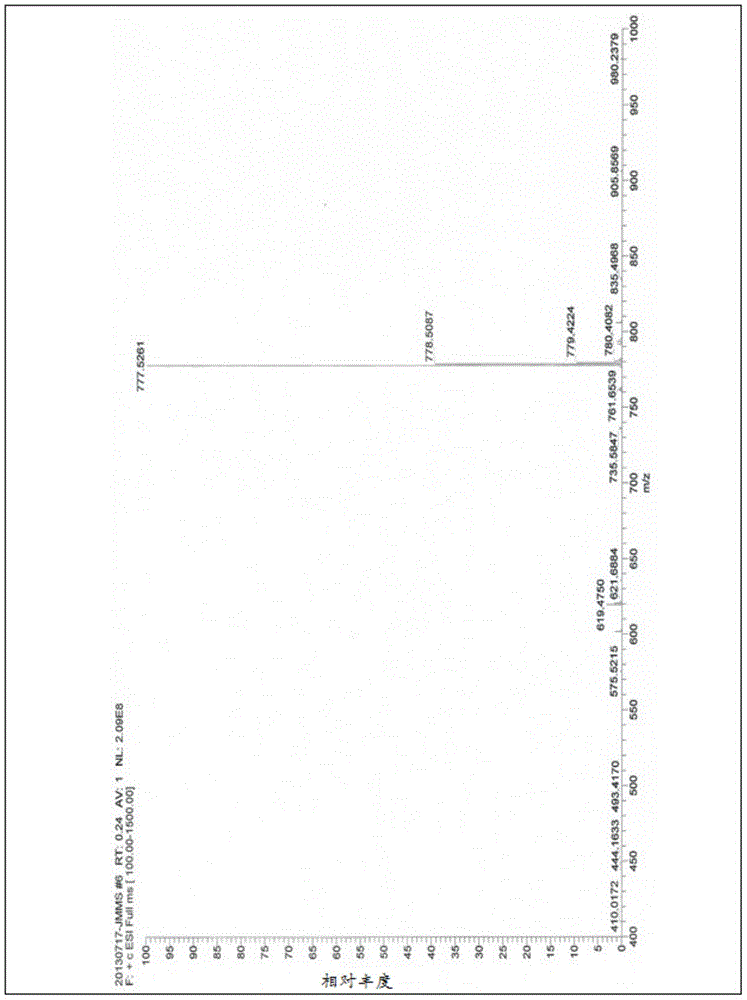
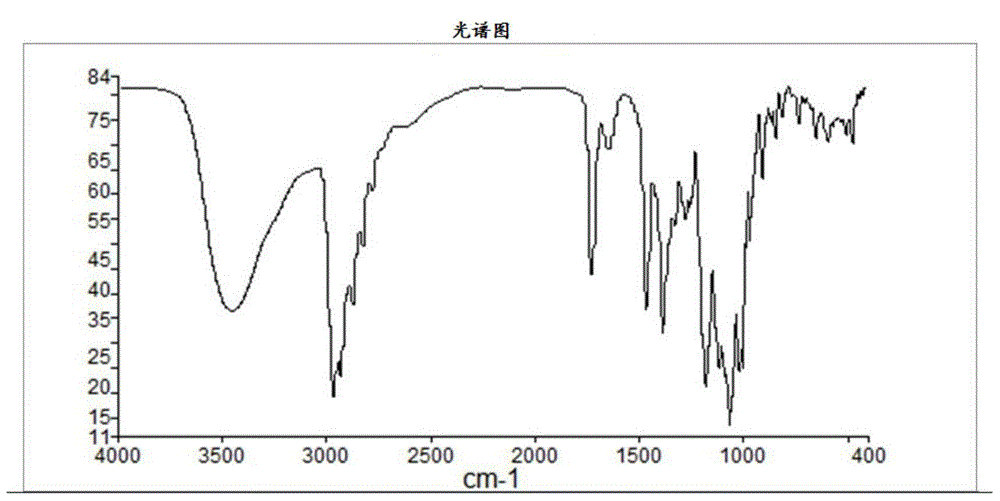
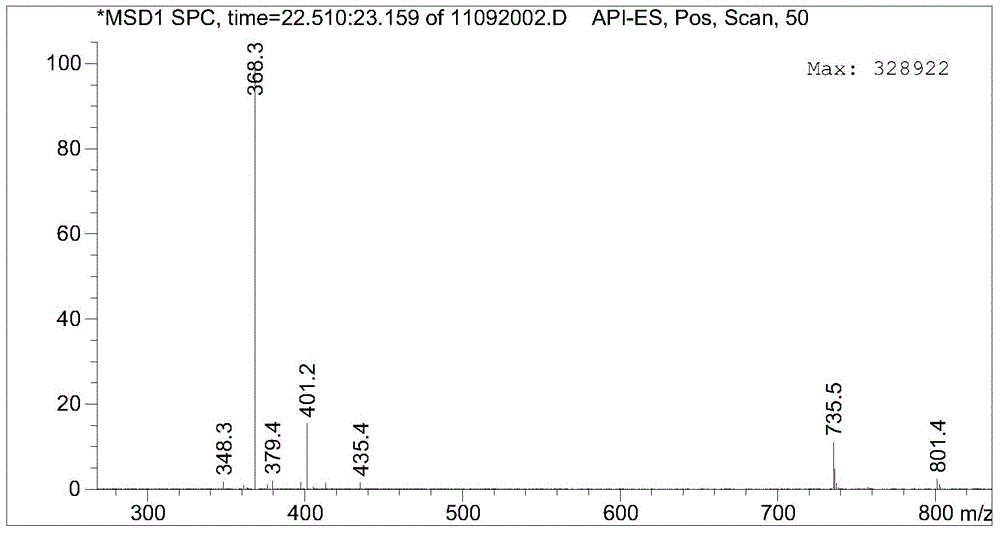
Gamithromycin preparation method
A technology of garamimycin and erythromycin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult scale-up production, high cost, and high production cost, and achieve easy scale-up production, The reaction process is mild and the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Preparation of 9(E)-9-deoxy-erythromycin A oxime:
[0055] Add 100g of erythromycin and 300ml of MeOH (methanol) into a 1000ml three-necked flask, stir to dissolve, add 76.6g of triethylamine, heat to 50°C-60°C for 0.5h, add 43.8g of hydroxylamine hydrochloride, and heat to reflux for 1h- 3h, TLC was used to detect the completion of the reaction. After the reaction was completed, 200 ml of water was added, and the precipitate was filtered off to obtain 106 g of crude product 9(E)-9-deoxy-erythromycin A oxime. The crude product was mixed with 300ml isopropanol and 100g ammonia water (content is expressed as NH 3 Calculated as 25%) recrystallization to obtain 80.6g of the product, the molar yield was 83.3%, and the HPLC detection purity was 96.5%. The melting point (mp) is 156°C-160°C.
[0056] HPLC detection conditions: Agilent ZORBAX SB-C18, 5μm4.6*150mm, phosphate solution (take 8.7g of dipotassium hydrogen phosphate, add 1000ml of water, adjust the pH value to ...
Embodiment 2
[0066] A preparation method of gamithromycin, comprising the following steps:
[0067] (1) Synthesis of 9(E)-9-deoxy-9-hydroxyiminoerythromycin A from erythromycin A (hereinafter referred to as 9(E)-9-deoxy-erythromycin A oxime):
[0068] Under the condition of 50-70°C, triethylamine is used as an acid-binding agent, and 100g of erythromycin A is reacted with hydroxylamine hydrochloride in methanol (analytically pure) to obtain 9(E)-9-deoxy-erythromycin A, wherein the molar ratio of erythromycin to hydroxylamine hydrochloride is 1:3, and the molar ratio of triethylamine to erythromycin A is 1:3;
[0069] The reaction product is recrystallized with isopropanol and ammonia water. The volume-to-mass ratio of isopropanol to erythromycin A is 5:1 in ml / g. The ratio is 2:1;
[0070] (2) Synthesis of 9(Z)-9-deoxy-9-hydroxyiminoerythromycin A from 9(E)-9-deoxy-erythromycin A oxime (hereinafter referred to as 9(Z)-9- Deoxy-erythromycin A oxime):
[0071] Under the condition of 60℃,...
Embodiment 3
[0082] A preparation method of gamithromycin, comprising the following steps:
[0083] (1) Synthesis of 9(E)-9-deoxy-9-hydroxyiminoerythromycin A from erythromycin A (hereinafter referred to as 9(E)-9-deoxy-erythromycin A oxime):
[0084] Under the condition of 50-70°C, triethylamine is used as an acid-binding agent, and 100 g of erythromycin A is oximated with hydroxylamine hydrochloride in isopropanol to obtain 9(E)-9-deoxy-erythromycin A, Among them, the molar ratio of erythromycin to hydroxylamine hydrochloride is 1:6, and the molar ratio of triethylamine to erythromycin A is 1:7;
[0085] The reaction product is recrystallized with isopropanol and ammonia water. The volume to mass ratio of isopropanol to erythromycin A is 3:1 in ml / g. The ratio is 0.5:1;
[0086] (2) Synthesis of 9(Z)-9-deoxy-9-hydroxyiminoerythromycin A from 9(E)-9-deoxy-erythromycin A oxime (hereinafter referred to as 9(Z)-9- Deoxy-erythromycin A oxime):
[0087] 9(E)-9-deoxy-erythromycin A oxime is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com