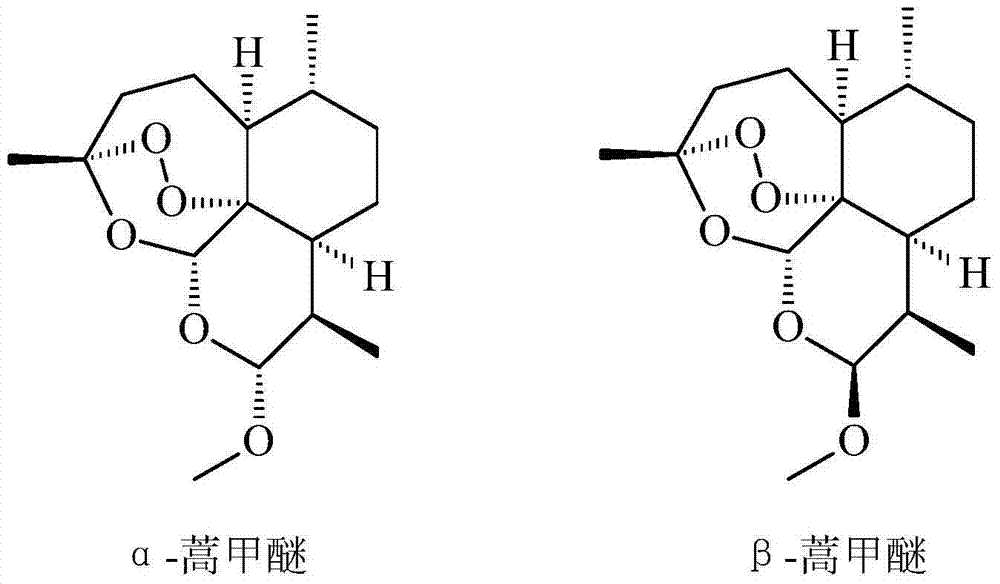
Preparation process of artemether
A preparation process, technology of artemether, applied in the field of preparation of high-purity β-artemether, can solve the problems of low content of β-artemether, complex operation, etc., to achieve recycling, short production cycle, and energy saving Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 100g of dihydroartemisinin, put it into a 2000ml three-necked bottle, and add 1000ml of dimethyl carbonate and 5ml of boron trifluoride methyl ether successively under stirring. React at room temperature for 2 hours, take a sample for thin-layer chromatography (TLC) to detect no dihydroartemisinin spots, that is, dihydroartemisinin has completely reacted. The pH of the solution was adjusted to 7 by adding saturated sodium bicarbonate solution. The reaction solution was concentrated to dryness at 50°C under vacuum to obtain 98.23 g of artemether crude crystals. Artemether coarse crystals were dissolved in methanol, crystallized at low temperature, and dried at 50°C to obtain the artemether product, which contained 95.16 g of β-artemether through detection and calculation.
Embodiment 2
[0031] Weigh 100g of dihydroartemisinin, put it into a 2000ml three-necked bottle, and add 1500ml of dimethyl carbonate and 5ml of boron trifluoride methyl ether successively under stirring. React at room temperature for 2 hours, and take a sample TLC to detect no dihydroartemisinin spots. The pH of the solution was adjusted to 7 by adding saturated sodium bicarbonate solution. The reaction solution was concentrated to dryness at 50°C under vacuum to obtain 98.46 g of artemether crude crystals. Artemether coarse crystals were dissolved in methanol, crystallized at low temperature, and dried at 50°C to obtain the artemether product, which contained 95.32 g of β-artemether through detection and calculation.
Embodiment 3
[0033] Weigh 100g of dihydroartemisinin, put it into a 2000ml three-necked bottle, and add 1000ml of ethyl acetate and 10ml of boron trifluoride methyl ether successively under stirring. After reacting at room temperature for 1.5 hours, no dihydroartemisinin spots were detected by sampling TLC. The pH of the solution was adjusted to 7 by adding saturated sodium bicarbonate solution. The reaction solution was concentrated to dryness at 50°C under vacuum to obtain 98.36 g of artemether crude crystals. Artemether coarse crystals were dissolved in methanol, crystallized at low temperature, and dried at 50°C to obtain the artemether product, which contained 95.25 g of β-artemether through detection and calculation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com