Industrialized preparation method for capecitabine
A technology of capecitabine and its synthesis method, which is applied in the field of preparation technology and product purification of antineoplastic drug capecitabine raw materials, and can solve the problems of low purity of capecitabine raw materials and breakage of amide bonds in capecitabine , slow dissolution of crude capecitabine, etc., to achieve the effect of reducing operational complexity, reducing the difficulty of purification, and strict temperature control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
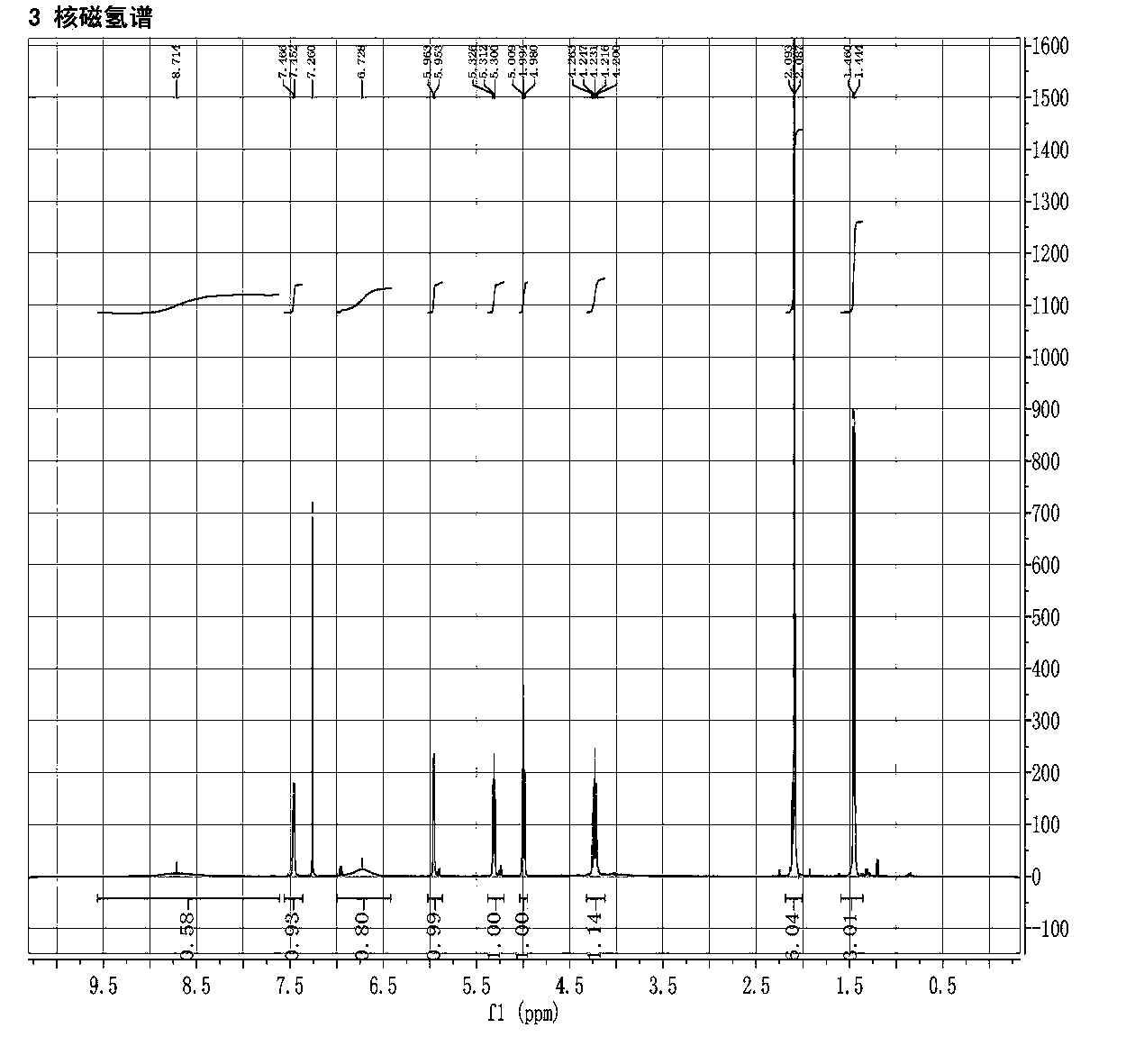
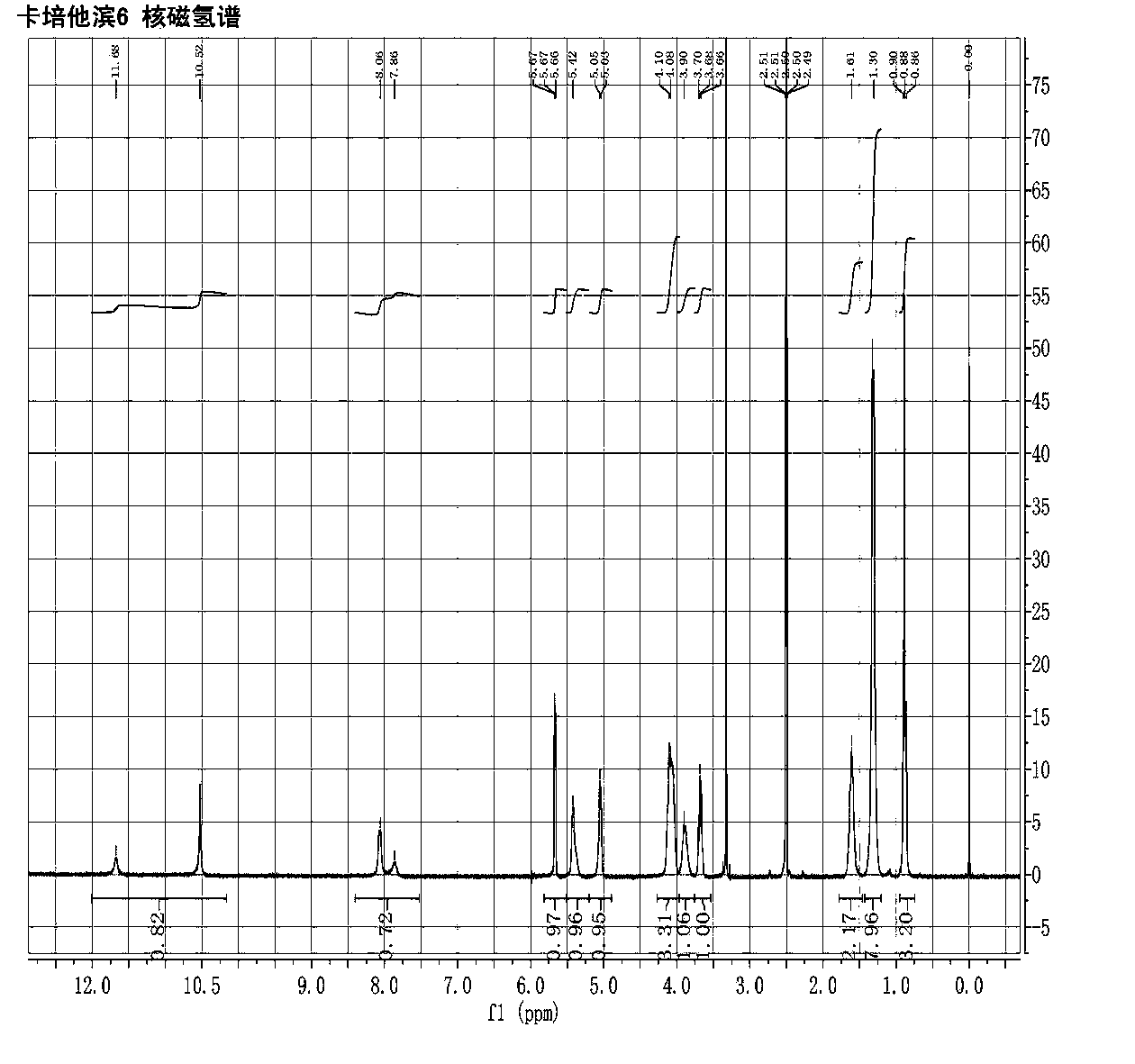
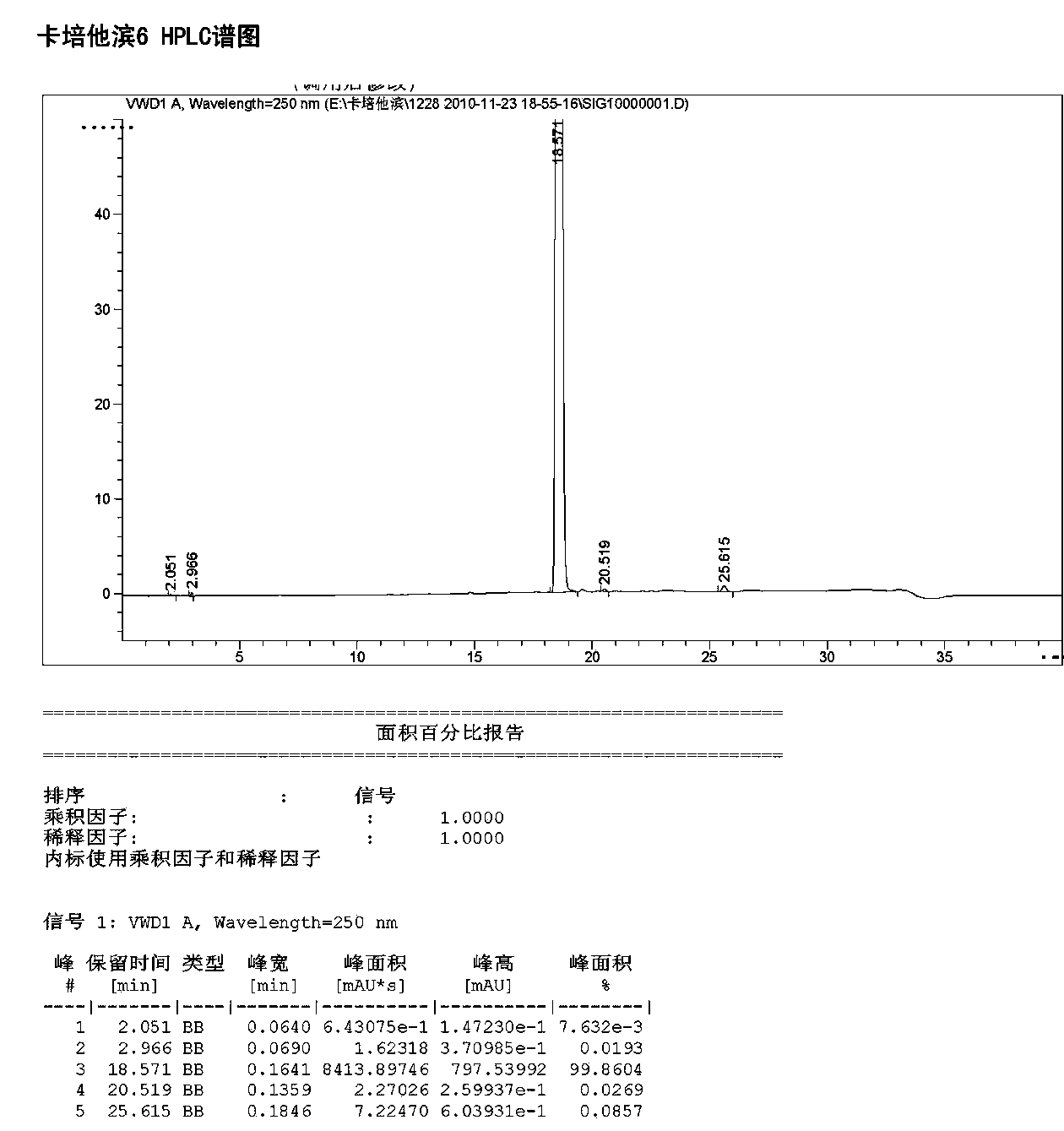
Image
Examples
Embodiment 1
[0031] 14.2 kg (110 mol, 1.1eq) 5-fluorocytosine 1 Added to 50 L reactor, then added 29.8 L HMDS (143 mol, 1.43eq), then added 20 L toluene, stirred and heated to reflux for 4h. Decompression (rotary evaporator bath temperature is not higher than 70 o C) The solvent was removed to obtain a white solid powder.
[0032] The above white solid powder and 5-deoxy-1,2,3-triacetyl deoxyribose 2 26 kg (100 mol, 1eq) was suspended in 100 L of dichloromethane, and the temperature in the reactor was lowered to below 2 oC 15.2 L (130 mol, 1.3eq) SnCl was added dropwise with a constant pressure funnel 4 10 L of dichloromethane solution (inside temperature range of 2 oC to 8 o C), slowly return to room temperature after dropping, continue to stir and react at room temperature for 12 h, then add 97.5 kg of anhydrous Na to the system at low temperature 2 CO 3 (920 mol, 9.2eq), then slowly add H 2 O 26 L (keep the internal temperature not higher than 25 oC ), keep the internal...
Embodiment 2
[0036] Compound 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine 3 19.76 kg (60 mol, 1 eq) was dissolved in 49.4 L of dichloromethane (1 kg : 2.5 L), and 9.66 L of pyridine (120 mol, 2 eq) was added, and the inner temperature of the reactor dropped to -15 o Below C, start to add n-amyl chloroformate dropwise 4 12.17 L (84 mol, 1.4 eq), while keeping the internal temperature below -5 O c. After the dropwise addition, the temperature was naturally restored and the reaction was monitored by TLC until the reaction was complete. Add 20 L H 2 O and 50 L of dichloromethane were stirred for 10 min, and then the layers were separated; the organic phase was separated and washed with water (20 L*2); the aqueous phase was back-extracted with dichloromethane (50 L*2); the organic phases were combined , dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to give yellow oily product 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine 5 (d...
Embodiment 3
[0038] The crude product 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine obtained in the previous step 5 Dissolve in 28 L of methanol, and wait until the temperature in the reactor drops to -10 o Below C, start adding 9.6 kg NaOH (240 mol, 4 eq) dropwise in 24 L H 2 O solution (10 mol / L), during which the internal temperature should not be higher than -5 o C, continue to keep the low temperature after dripping (-5 o C) to react for 20 min, then add about 20 L of concentrated hydrochloric acid dropwise, adjust the pH to 4-5, and keep the internal temperature not higher than -5 o C. Add 10 L of water and 100 L of dichloromethane after the dropwise addition, let stand to separate the layers, and separate the organic phase. The organic phase was washed with water (20 L*2). The aqueous phase was back-extracted with dichloromethane (50 L*2). Combined organic phases, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to give a slightly yellow o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com