Patents
Literature
40 results about "Capecitabina" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
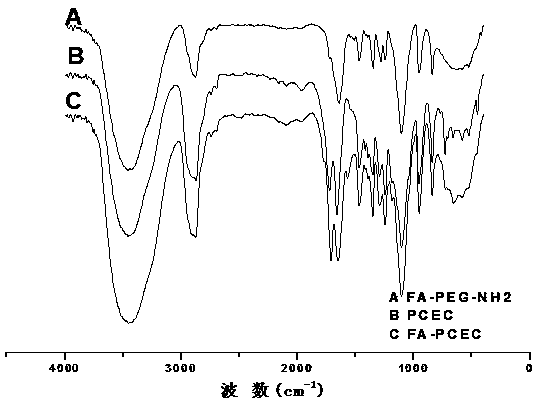
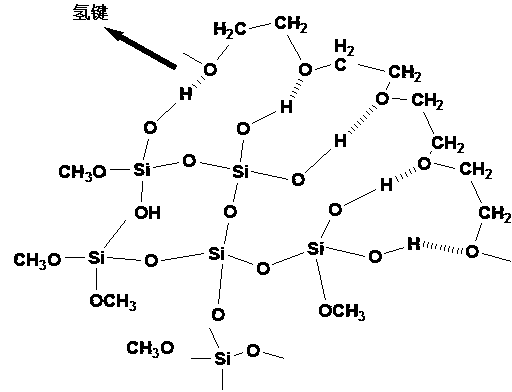
Targeting and slow-release antineoplastic medicine nanoparticle with amphiphilic polyurethane as carrier and preparation method thereof
ActiveCN103751148AFlat shapeUniform sizeOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereTherapeutic effect
The invention discloses a trgeting and slow-release antineoplastic medicine nanoparticle with amphiphilic polyurethane as a carrier and a preparation method thereof. The nanoparticle is of a nucleus-shell structure provided with two shell layers; a hydrophilic section of amphiphilic polyurethane serves as the shell layer, and a hydrophobic section serves as the nucleus; the nucleus covers the medicines; a functional molecule exposes from the surface of the shell layer of the nanoparticle; organo-siloxane can be hydrolyzed to form another shell layer between the hydrophilic section and the hydrophobic section; the medicines include capecitabine, adriamycin and paclitaxel; the functional molecule is folic acid; and organo-siloxane is tetramethoxysilane. The targeting and slow-release antineoplastic medicine nanoparticle with amphiphilic polyurethane as the carrier has the characteristics that degradable polymer materials are used as the medicine release-control preparations, which enables the medicine to act on a specified wound position with the minimum dosage; and the medicine release rate can be optimized to improve the treatment effect as well as reduce the toxic and side effects.
Owner:广州智园生物科技有限公司
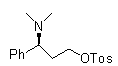
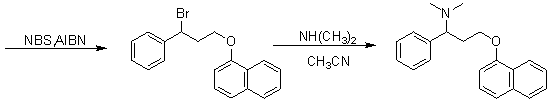
Novel synthesizing method of dapoxetine
InactiveCN103304434AHigh yieldHigh stereoselectivityGroup 4/14 element organic compoundsOrganic compound preparationHydroxylaminePtru catalyst
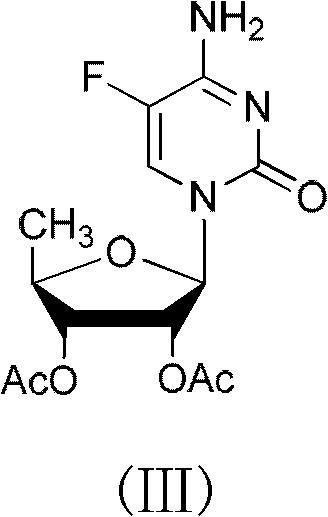
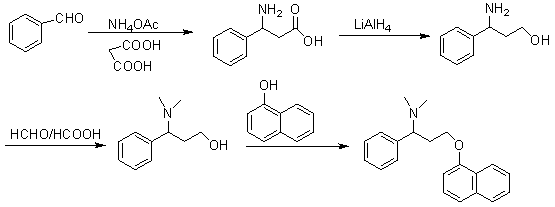
The invention discloses a novel synthesizing method of dapoxetine, wherein a series of reactions are carried out on trans-cinnamaldehyde and N-carbobenzoxy hydroxylamine which serve as initiative raw materials under the action of a self-prepared catalyst (s)-alpha-alpha-diisopropyl dimethyl tert-butyl silicon oxygroup prolinol, so that an important intermediate of the dapoxetine, namely an important chiral intermediate (S)-3-amino-3-phenyl propyl alcohol (compound 4), is obtained. The invention further discloses a preparation method of capecitabine, wherein the compound serving as a raw material is methylated and protected by hydroxyl and then reacts with 1-naphthol to form a salt, so that the capecitabine is obtained. The preparation method is easy in raw material obtainment, good in stereoselectivity and high in yield, thereby being suitable for industrial production.
Owner:湖南欧亚药业有限公司
Paclitaxel and diazepinocarbazole compound combined pharmaceutical composition
InactiveCN108578412AAdvantages and Notable ImprovementsSignificant progressOrganic active ingredientsAntineoplastic agentsSide effectBULK ACTIVE INGREDIENT
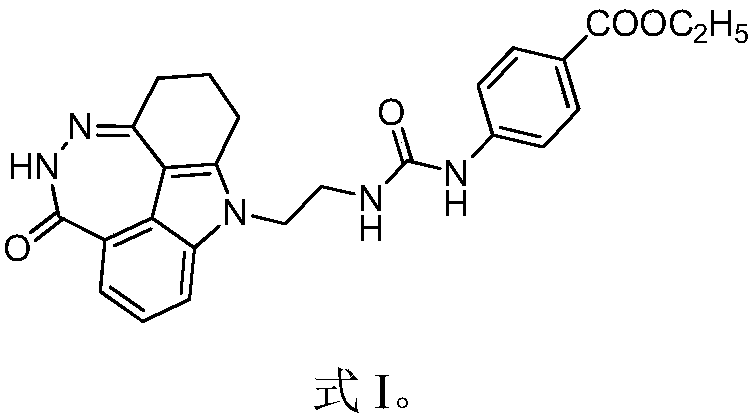
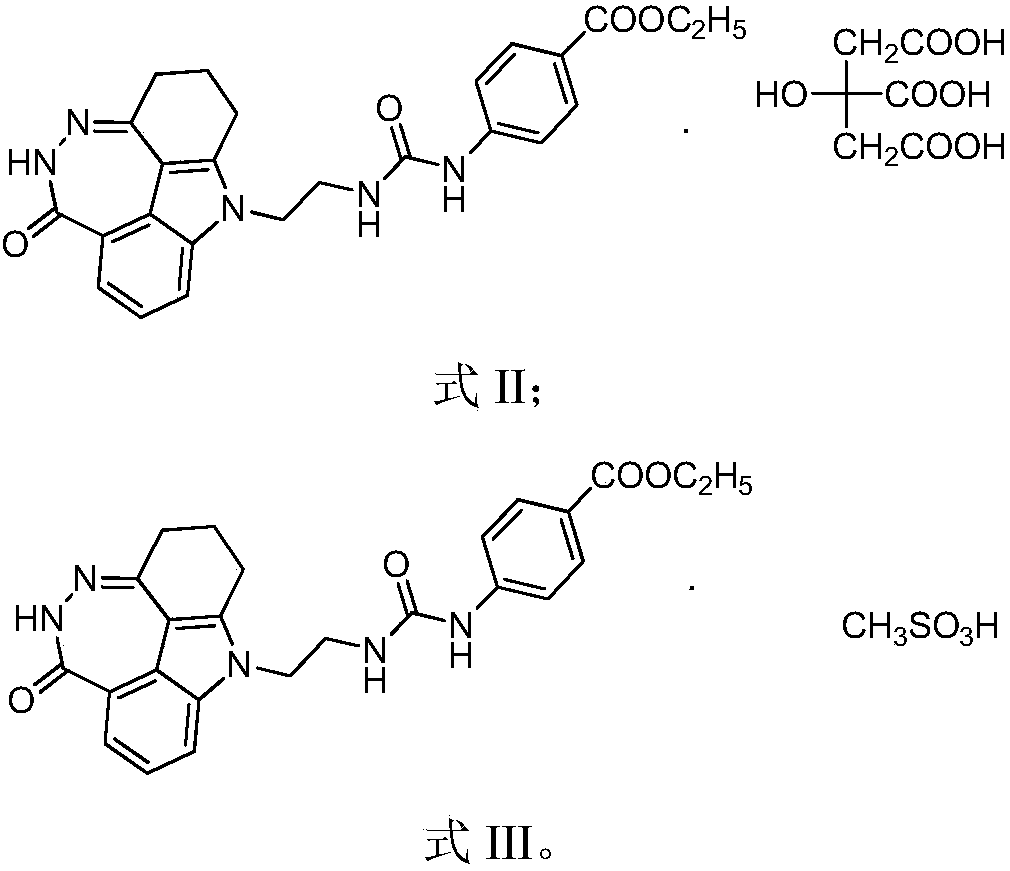
The invention provides a paclitaxel and diazepinocarbazole compound combined pharmaceutical composition, which comprises active ingredients and pharmaceutically acceptable excipients. The paclitaxel and diazepinocarbazole compound combined pharmaceutical composition is characterized in that the active ingredients are composed of gemcitabine and a diazepinocarbazole compound as shown in the formulaI or pharmaceutically acceptable salts thereof; and mass ratio of gemcitabine to the diazepinocarbazole compound or the pharmaceutically acceptable salts in the active ingredients is (2-8): 1. The pharmaceutical composition has good anticancer curative effect but low toxic or side effect. As the diazepinocarbazole compound is sensitive to gemcitabine, the diazepinocarbazole compound and gemcitabine are combined to produce a synergistic effect. Thereby, clinical dosage of capecitabine is reduced, toxic or side effect caused by large dosage of capecitabine is decreased, and safety index of clinic treatment is raised. The paclitaxel and diazepinocarbazole compound combined pharmaceutical composition has a good clinical application prospect.
Owner:南京众慧网络科技有限公司
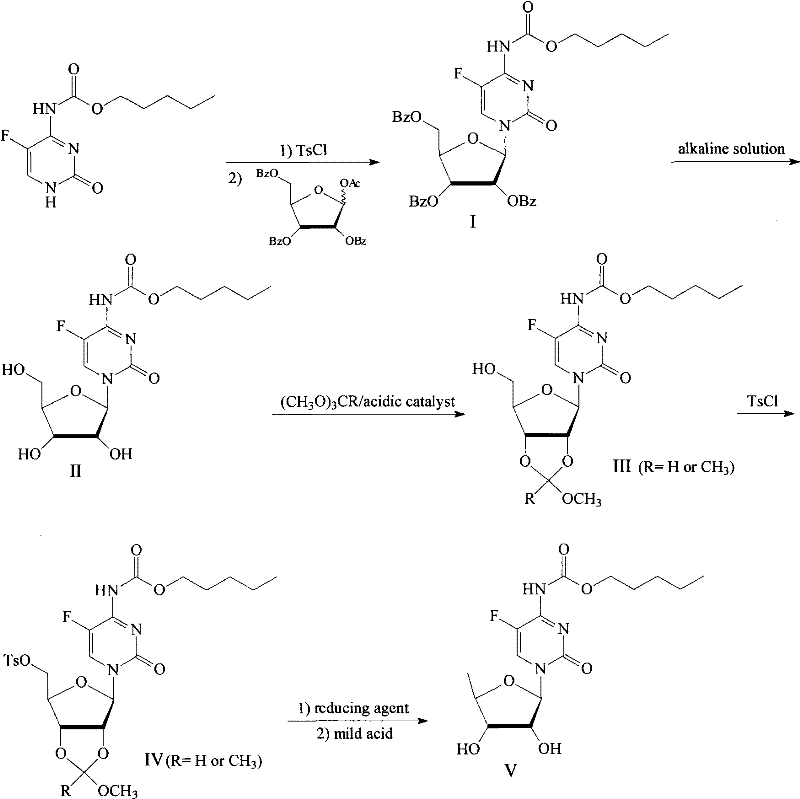
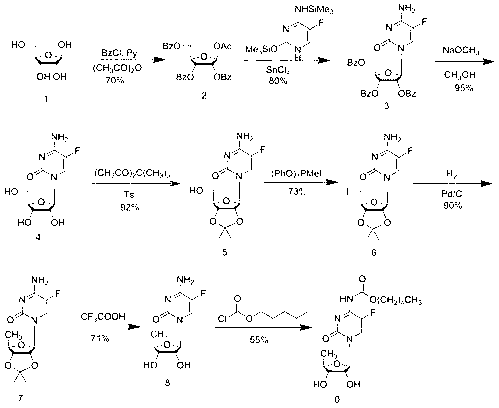
Method for preparing capecitabine and hydroxyl derivative intermediate thereof
InactiveCN102241721AMild reaction conditionsSimple processSugar derivativesSugar derivatives preparationChemical reactionControllability
The invention discloses a method for preparing an anticancer medicine capecitabine and a hydroxyl derivative key intermediate thereof, and belongs to the technical field of pharmaceutical chemistry. The method is characterized in that: N-[(p-pentyloxy)carbonyl]-5-flurocytosin is taken as an initial raw material, and is subjected to five steps of chemical reactions to form the capecitabine. The preparation method has the advantages of reasonable sequence, readily available raw materials, mild reaction conditions, high process controllability, high yield and low cost. The crude intermediate has high purity, complicated purification treatment is avoided, the capecitabine obtained in a later stage can reach the standard of United States Pharmacopeia, and the method is more suitable for industrial production.
Owner:JIANGNAN UNIV
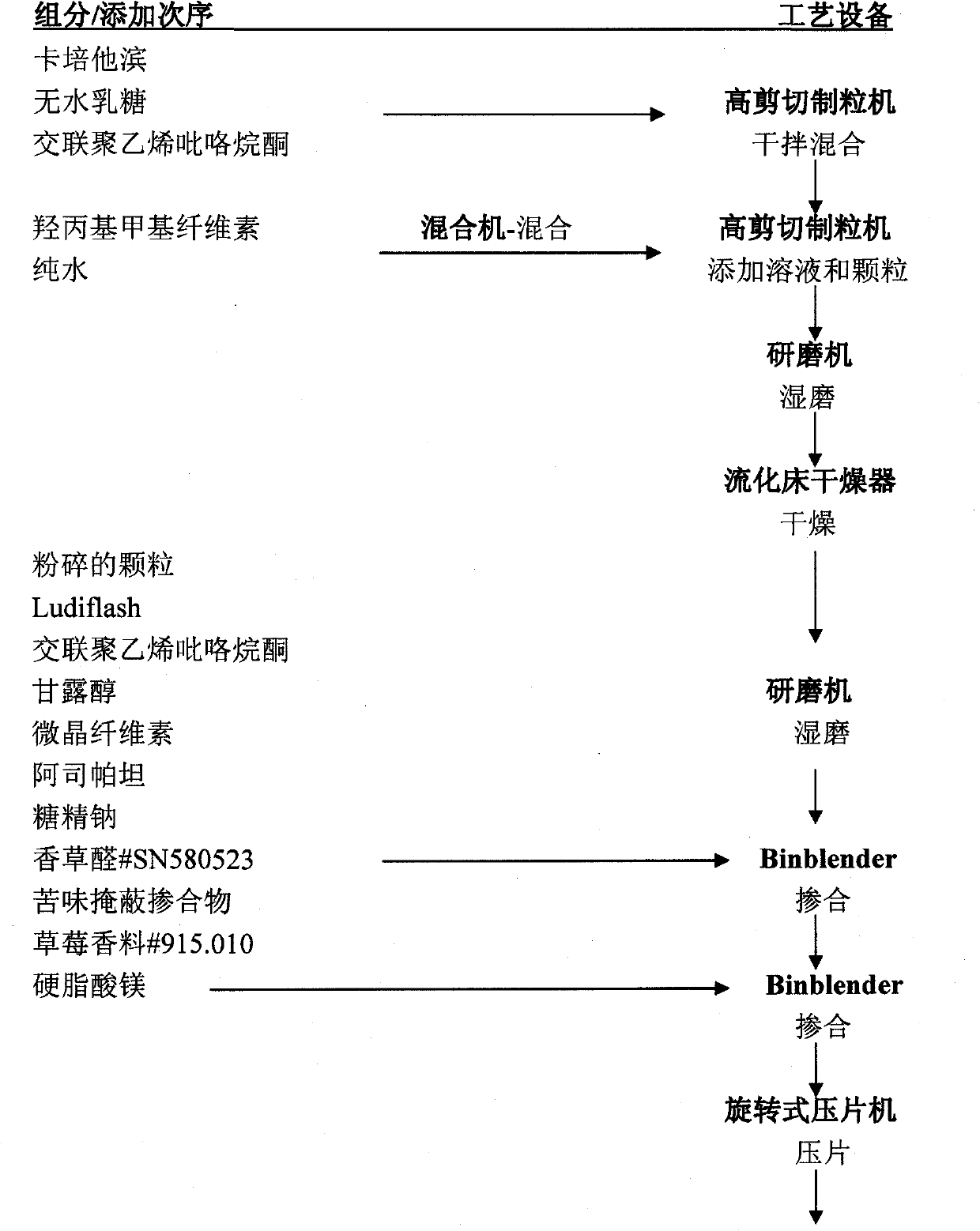
Capecitabine rapidly disintegrating tablets
InactiveCN102369002AOrganic active ingredientsDispersion deliveryCroscarmellose sodiumCarboxymethylcellulose Sodium
There is provided a film coated pharmaceutical composition comprising 5 '-deoxy-5-fluoro-N-[(pentyloxy)-carbonyl]-cytidine (capecitabine) and at least one disintegrant selected from the group comprising of crospovidone (particle size < 15-400 [mu]), croscarmellose sodium, sodium starch glycolate, low-substituted hydroxypropylcellulose, Ludiflash TM or any combination of these, together with other pharmaceutically acceptable excipients to form a rapidly disintegrating tablet.
Owner:F HOFFMANN LA ROCHE & CO AG
Novel technology for synthesis of capecitabine
InactiveCN103288905ASuitable for industrialized mass productionHigh yieldSugar derivativesSugar derivatives preparationSodium methoxideMeth-
The invention relates to a novel technology for synthesis of capecitabine. The technology is characterized in that: 5-fluorocytosine protected by trimethyl silicon is taken as raw material; and the capecitabine is obtained after condensation, esterification and deacetylation. The Reaction sequence is more economically reasonable, the synthetic route is short, the cost is low, the operation is simplified, the yield is high, the synthetic period is short, the quality of intermediates can be controlled, solvents used in reaction are few, pollution to the environment is little, and the technology is suitable for industrial production. Comparing the technology with the prior art for capecitabine production, trimethylsilyl trifluoromethanesulfonate (TMSOTf) which replaces a heavy metal agent stannic chloride is used as a condensing agent for glycosylation (condensation), and a sodium methoxide / methanol system replaces an ammonia gas / methanol system for deacetylation, so that the production yield is increased, and heavy metal residues of the products and the environmental pollution are reduced. The overall yield of the technology of the invention reaches 59%, the purity of the production is high and meets the standards of the United States Pharmacopeia.
Owner:北京博时安泰科技发展有限公司
Synthetic method for capecitabine key intermediate
ActiveCN104926901AHigh yieldControl ratioSugar derivativesSugar derivatives preparationChloroformateChloroform
The invention discloses a synthetic method for capecitabine key intermediate 2`,3`-O-diacetylpyridine-5`-deoxygenation-5-fluorine-N4-[(pentyloxy) carbonyl] cytidine. The synthetic method for the capecitabine key intermediate comprises the following steps that 1, 5- fluorocytosine, an acid-binding agent, chloroform, water and phase transfer catalyst are mixed, pentyl chloroformate is added dropwise under stirring, and the chloroform solution of (5-fluorine-2-oxo-1,2-dihydropyrimidine-4-base) amylcarbamate is obtained; 2, 1,2,3-three-O-acetyl-5-deoxygenation-6- ribofuranose is added into the chloroform solution obtained in the step 1, lewis acid is added dropwise, the reaction is performed for 2-10 hours after adding, and the capecitabine key intermediate is obtained after post-processing. The synthetic method is simple and convenient in operation, a silicane protective agent and intermediate product purification are not needed, the high yield of finished products is achieved, the proportion of alpha isomer in the products is effectively controlled, and compared with literature data, the purity of the obtained products is greatly improved.
Owner:广安凯特制药有限公司
Preparation method of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine
InactiveCN102250175ASugar derivativesSugar derivatives preparationTrimethylsilyl trifluoromethanesulfonate5-fluorocytidine
The invention provides a preparation method of capecitabine intermediate 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, which is suitable for actual industrial big production and has the advantages of high yield and quality and fine stability. The preparation method of capecitabine intermediate comprises the step of innovatively using a trifluoromethanesulfonic acid trimethylsilyl ester catalyst as the silanization agent of 5-fluorocytosine. The method has the advantages of high yield and good quality, and the process is simple and easy to operate.
Owner:NANJING VARSAL MEDICINE TECH DEV
Capecitabine pharmaceutical composition, and preparation method thereof
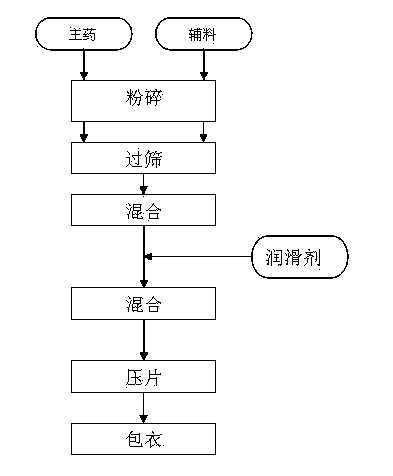
The invention relates to a capecitabine pharmaceutical composition, and a preparation method thereof, and belongs to the technical field of medicine. The preparation method of the capecitabine pharmaceutical composition comprises following steps: a prescription dosage of capecitabine, a filler, a dry adhesive, and a disintegrating agent are mixed; an obtained mixture is smashed using an air-flow type pulverizer, and is sieved; an obtained material is mixed using a mixer, a lubricant is added, and an obtained mixed material is mixed uniformly using the mixer, and is subjected to tabletting so as to obtain capecitabine tablets; a coating solution is prepared from a thin membrane coating composition with gastric solubility, and is used for spraying; and obtained coated capecitabine tablets are dried slowly so as to obtain capecitabine thin membrane coated tablets. According to the preparation method, powder direct tabletting method is adopted to produce the capecitabine thin membrane coated tablets; technical processes are simple and few; medicine stability is high; effects are achieved quickly, fully and effectively; irritation of the capecitabine tablets on esophagus can be reduced via thin membrane coating; medicine stability is increased via thin membrane coating; and related substances in the capecitabine thin membrane coated tablets are controlled strictly so as to ensure quality and pharmacy safety of the capecitabine pharmaceutical composition.
Owner:SUZHOU XINBEIRUI PHARMA
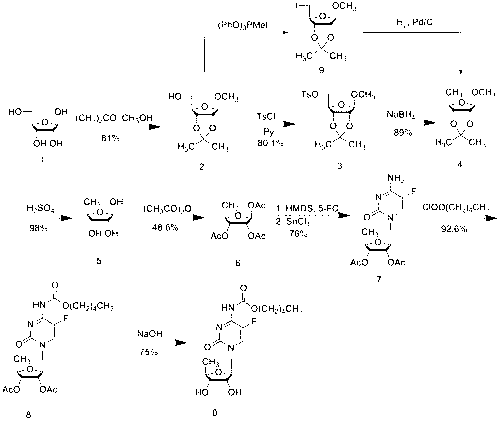
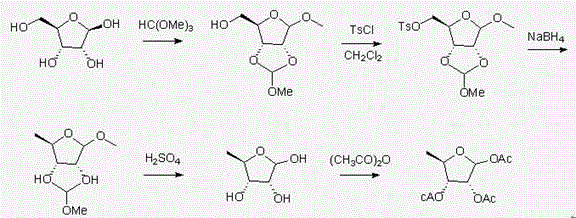
Novel synthesis method of capecitabine key intermediate 1,2,3-O-triacetyl-5-deoxy-D-ribose
InactiveCN104650160AHigh purityEasy to operateEsterified saccharide compoundsSugar derivativesTosylhydrazoneCatalytic effect
The invention discloses a novel synthesis method of a capecitabine key intermediate 1,2,3-O-triacetyl-5-deoxy-D-ribose. The synthesis method is characterized by comprising the following steps: reacting D-ribose which serves as an initial raw material with triethyl orthoformate under the catalytic action of acid, and realizing 2nd-site, 3rd-site and 4th-site hydroxyl protection in one step so as to obtain an intermediate product, namely 1-methyl-2,3-O-methoxymethylene-D-ribofuranose, and carrying out such steps as p-tosylation, hydrogenation reduction, deprotection and acylation so as to realize a new synthesis route of a target compound, namely 1,2,3-O-triacetyl-5-deoxy-D-ribose. The total yield of the route is 60% and product purity is 99.5%. The method disclosed by the invention has the advantages of original route design, high yield, good product purity, low production cost, safe and convenient operation, easy industrial production in a large scale and the like.
Owner:UNIV OF JINAN
Synthesis method of capecitabine
InactiveCN103193842AThe synthesis process is simpleReduce manufacturing costSugar derivativesSugar derivatives preparationPtru catalystTriflic acid
The invention relates to the technical field of synthesis of chemical medicines, and in particular relates to a synthesis method of capecitabine. The synthesis method of the capecitabine comprises the following steps: reacting 5-flucytosine with 5-deoxy-1,2,3-triacetoxy-D-ribose in the presence of a catalyst; performing aftertreatment on the reaction liquid to obtain 2',3'-diacetoxy-5'-deoxy-5-fluorocytidine; adding pentyl chloroformate dropwise into the 2',3'-diacetoxy-5'-deoxy-5-fluorocytidine and pyridine; and performing aftertreatment on the reaction liquid, adding alkaline liquid dropwise and performing treatment to obtain the capecitabine, wherein the catalyst comprises trifluoromethanesulfonic acid and derivatives thereof. The catalyst used in the method can use alpha, beta isomer mixed type 5-deoxy-triacetyl ribose as a raw material and does not need to separate ALFA and BETA isomers; and the generated ALFA type glucoside can be converted into BETA type glucoside, so the production process is simplified and the production cost is reduced.
Owner:JINING HIGH TECH DEV ZONE YONGFENG CHEM PLANT
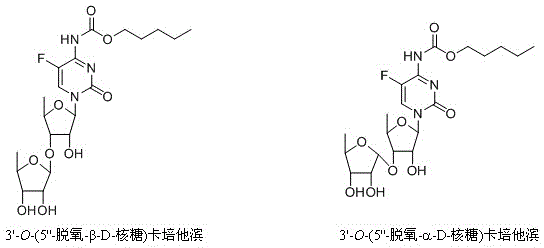
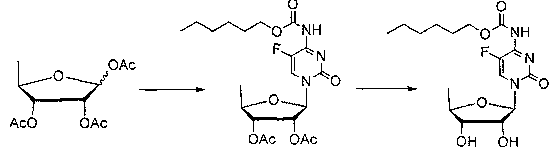
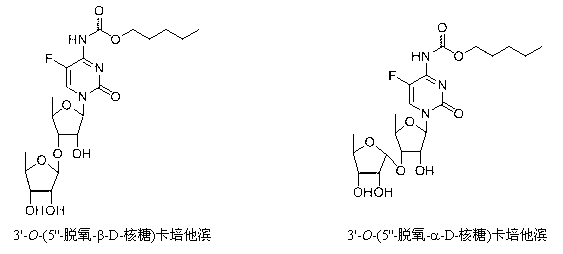
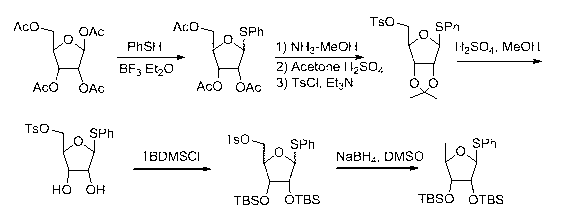
Preparation method of 5-deoxy-D-ribofuranose oxygen glycosides compound
The invention relates to a preparation method of substances 3'-O-(5''-deoxy-beta-D-ribose) capecitabine and 3'-O-(5''-deoxy-alpha-D-ribose) capecitabine relative to capecitabine medicines used for antineoplastic effect, which solves the problem that the impurity generated during a capecitabine production process can be separated from a reaction solution. The method is characterized in that a 5-deoxy-D-ribose donor is prepared, and is subjected to a glycosylation reaction with capecitabine protected by silane, and a protective group is removed to prepare the target product. A double glycosylation related substance can be generated during the production process of capecitabine prepared by a chemical method, and the method provides a qualified reference substance for controlling the quality of capecitabine.
Owner:SHENYANG PHARMA UNIVERSITY
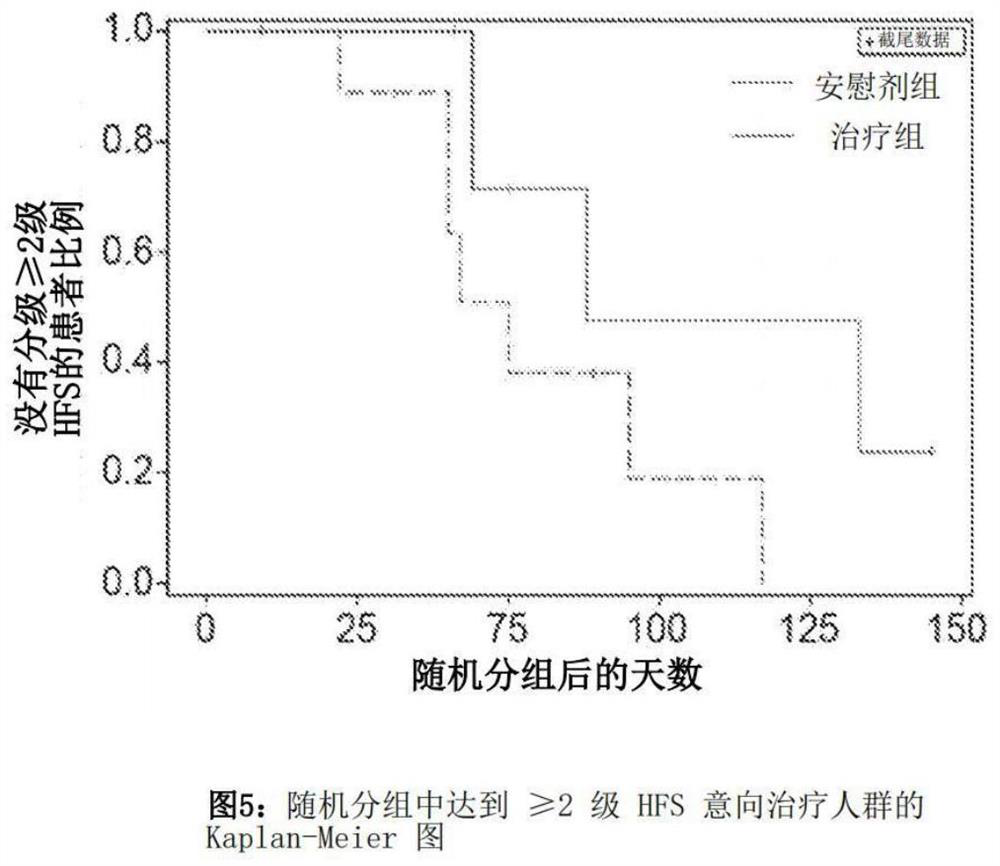
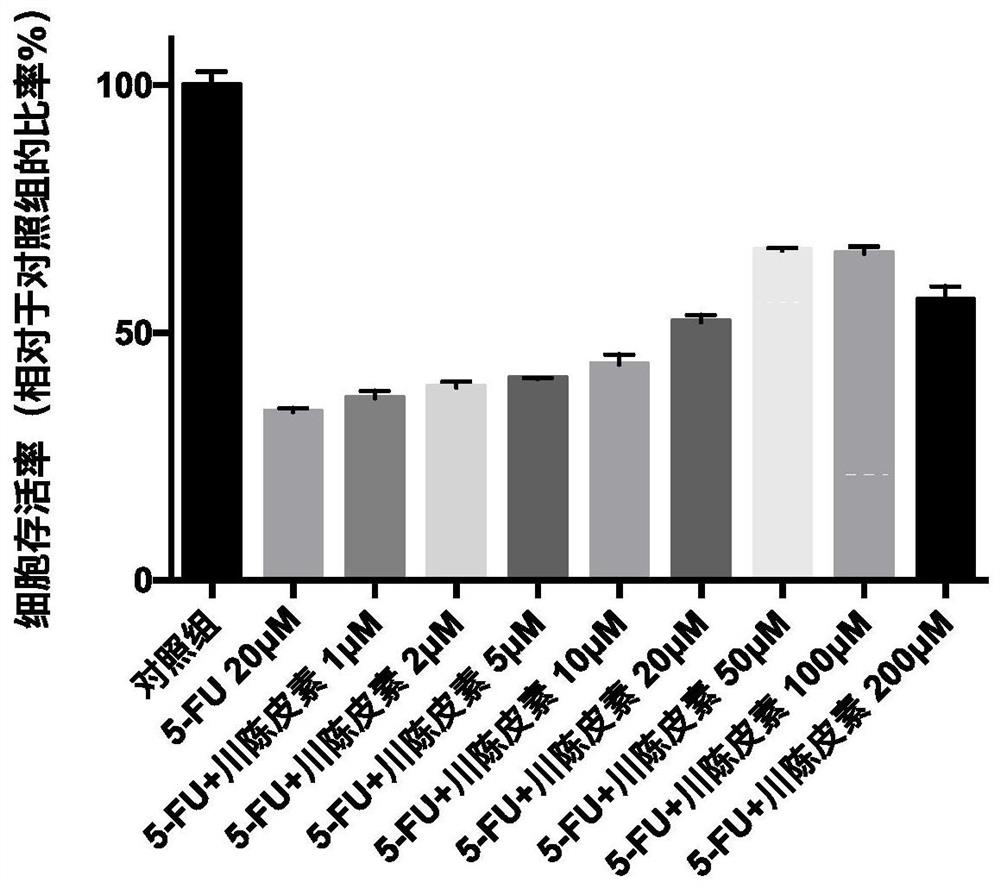
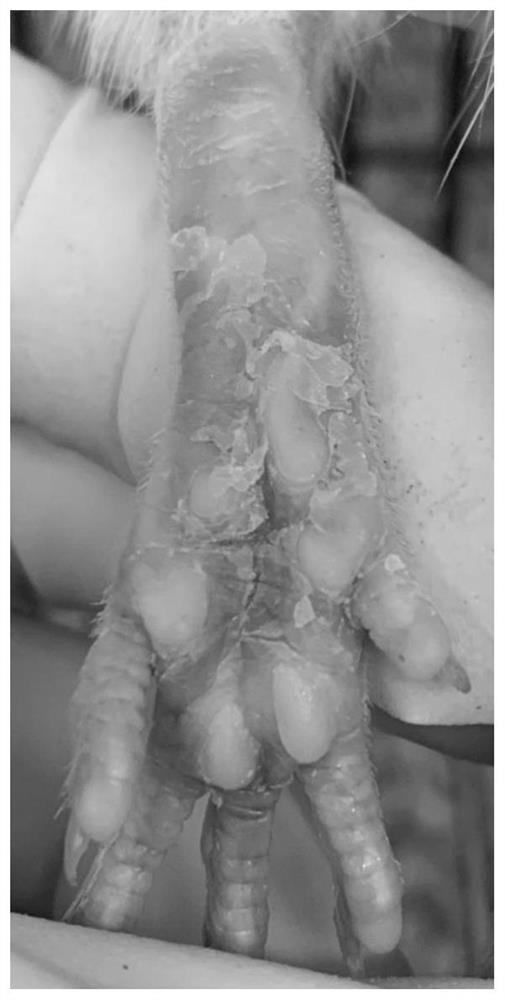
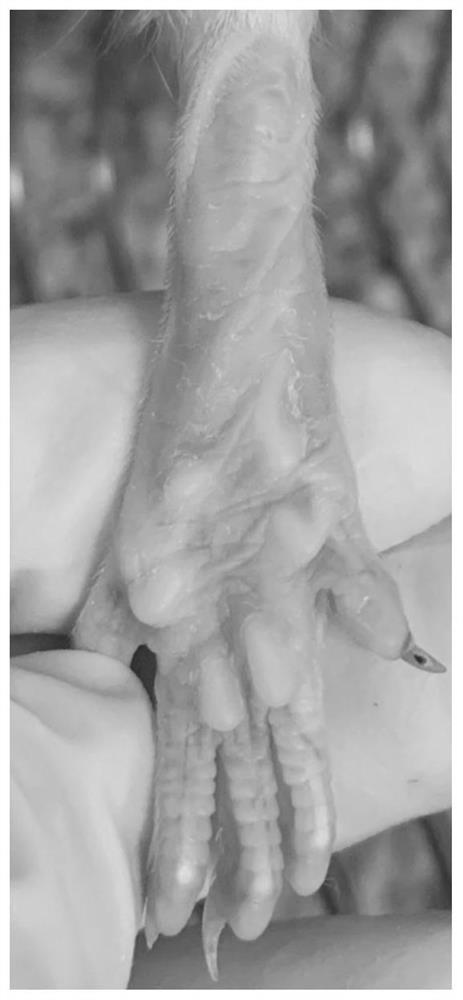
Application of nobiletin in preparation of medicines for preventing or treating skin tissue diseases or symptoms related to application of chemotherapeutics
PendingCN113116884AEffectively control side effectsPrevention of Hand-Foot Syndrome in RatsOrganic active ingredientsDermatological disorderDiseaseSide effect
The invention relates to an application of nobiletin in preparation of medicines for preventing or treating skin tissue diseases or symptoms related to application of chemotherapeutics. According to the application method disclosed by the invention, the nobiletin can be used for preventing or treating skin tissue diseases or symptoms related to chemotherapeutics administrated in subjects. The nobiletin can be used for effectively controlling skin side effects caused by chemotherapeutics. The invention discovers that the nobiletin can relieve negative influence of a prodrug 5-fluorouracil of capecitabine on cell proliferation at the cellular level. The nobiletin can significantly prevent capecitabine-induced hand-foot syndromes in rats at the animal level.
Owner:SHANGHAI JIAO TONG UNIV
Industrialized preparation method for capecitabine
ActiveCN103570781AStrict temperature controlLess side effectsSugar derivativesCombinatorial chemistryActive ingredient
The invention optimizes the synthesis process of capecitabine bulk drug, especially improves the purification method of capecitabine. The method involved in the invention is suitable for industrial production, remarkably reduces the quantity and limit of related impurities in the capecitabine bulk drug, and improves the quality of the capecitabine bulk drug.
Owner:SINOPHARM A THINK PHARMA
Tertiary amine-containing anthranilamide compound as well as preparation and application thereof
ActiveCN113200908ALow toxicityExcellent pharmacokinetic parametersOrganic chemistryAntineoplastic agentsApoptosisCancer research
The invention provides a tertiary amine-containing anthranilamide compound as well as preparation and application thereof. The tertiary amine-containing anthranilamide compound can inhibit proliferation, migration and invasion of gastric cancer cells and induce apoptosis, and has a cycle arrest effect. The tertiary amine-containing anthranilamide compound has higher activity than 5-fluorouracil on the cellular level, has higher activity than capecitabine on the animal level, is effective in oral administration, has lower toxicity than capecitabine, and is safer and more effective. The compound has good pharmacokinetic performance and can be applied to preparation of anti-gastric cancer drugs.
Owner:NANHUA UNIV
Method for preparing capecitabine intermediate 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine by enzymatic composite chemical method
The invention provides a preparation method of a capecitabine intermediate 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, which is suitable for actual industrial mass production and has the advantages of relatively high yield, quality and good stability. The preparation method of the capecitabine intermediate comprises the following steps: creatively converting substrates 5-fluorocytosine and 5-deoxyribose-1-phosphate to 5'-deoxy-5-fluoro-cytidine by using an enzyme catalyst pyrimidine nucleoside phosphorylase (EC2.4.2.2), and further performing acetylation reaction on the 5'-deoxy-5-fluoro-cytidine to obtain the 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine. The method has the advantages of high yield and good quality and is in line with environmental protection requirements; the process is simple and easy to operate.
Owner:北京六盛合医药科技有限公司
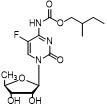
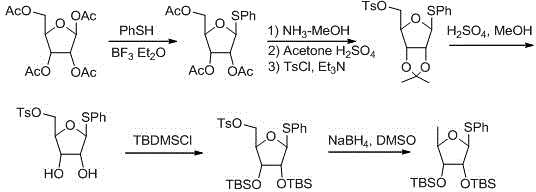
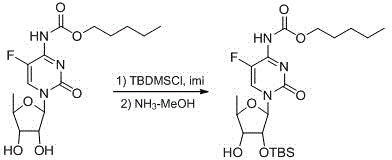
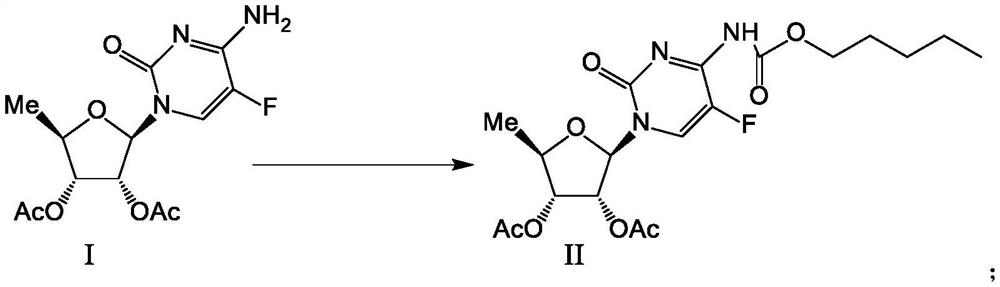
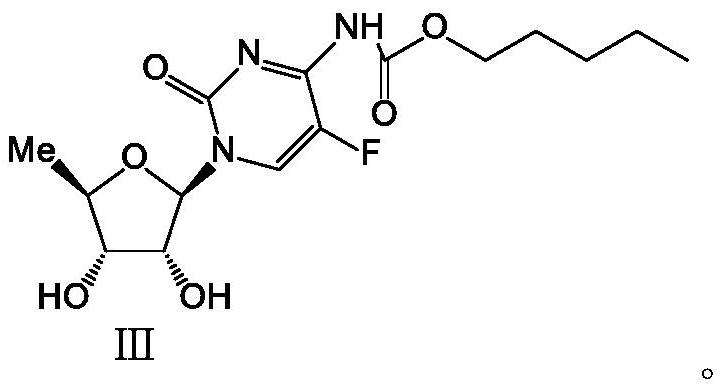
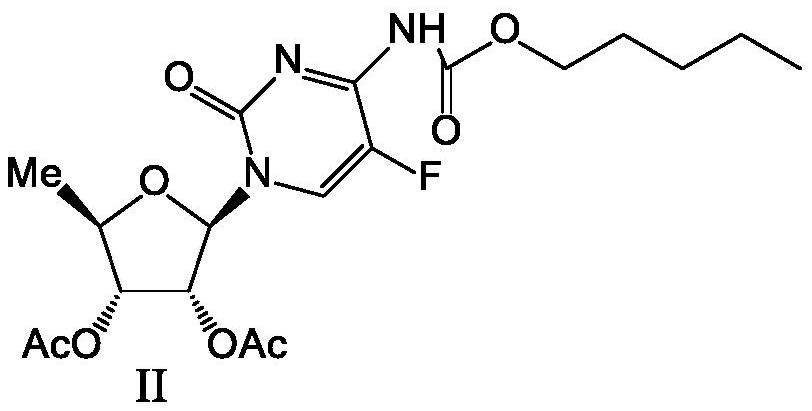
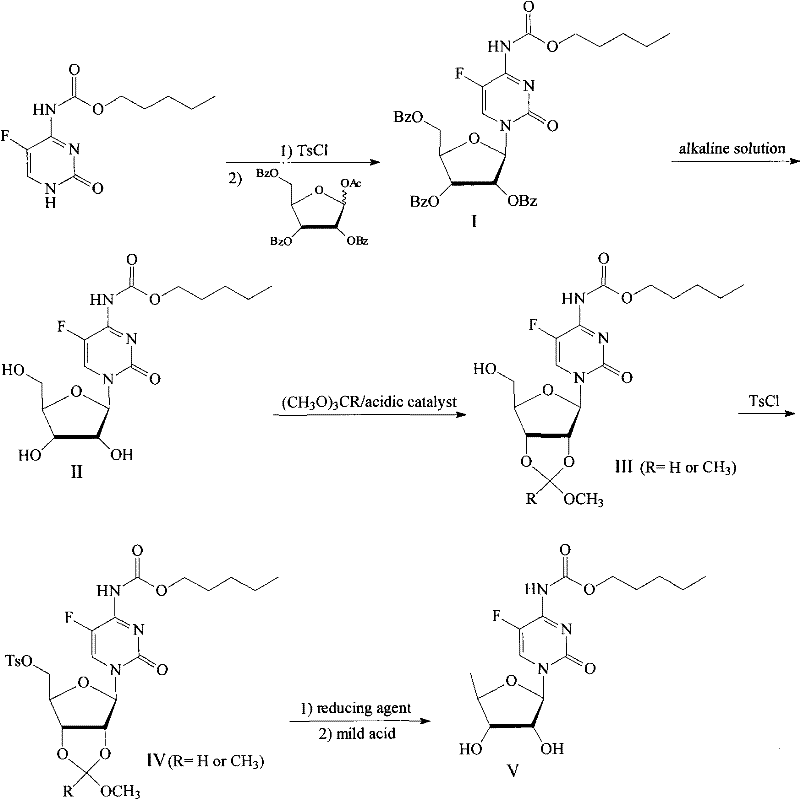
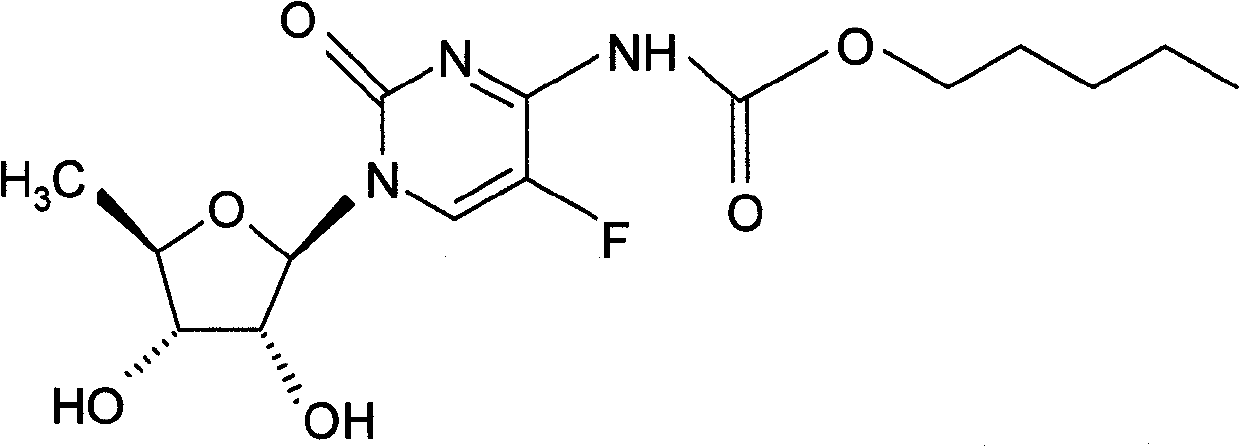
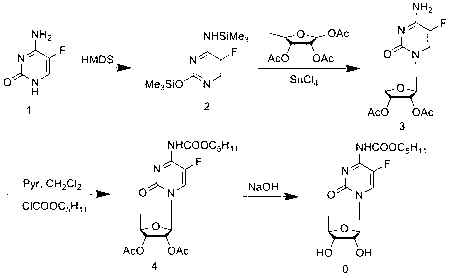
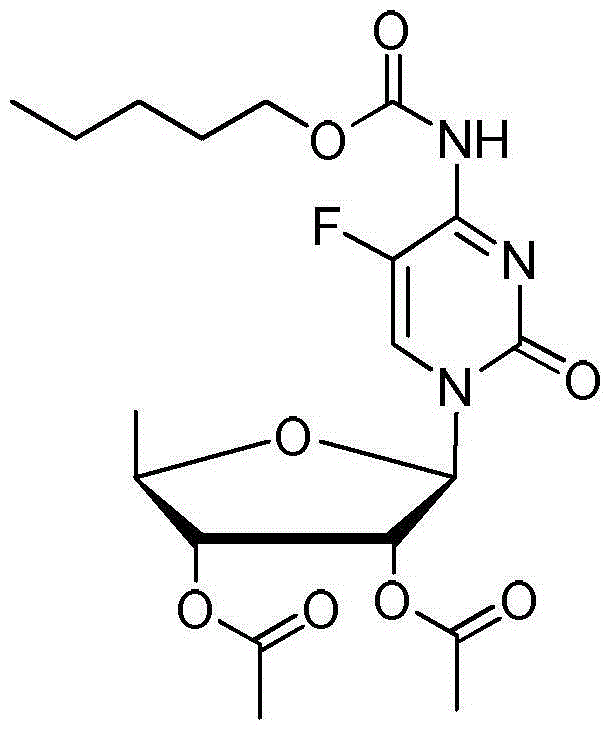
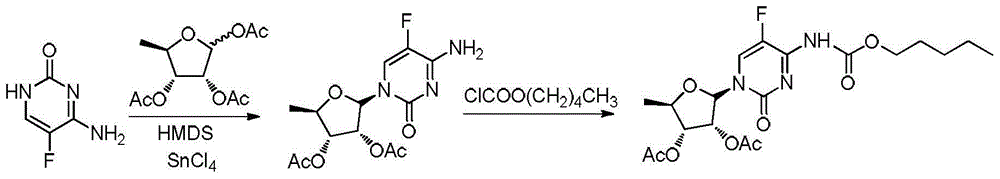
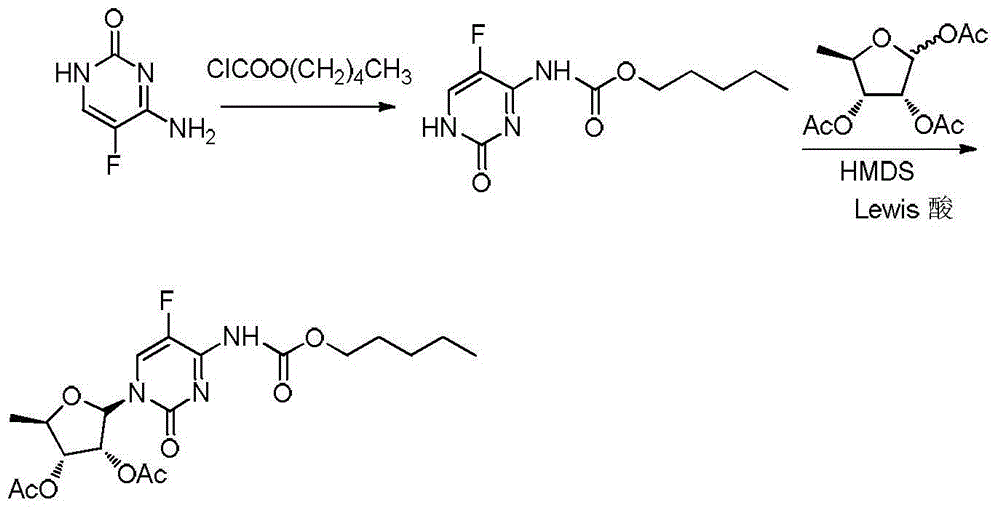
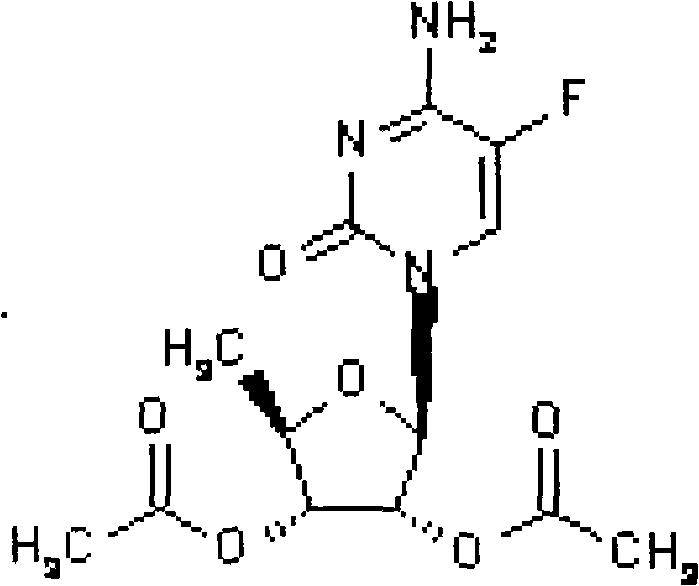
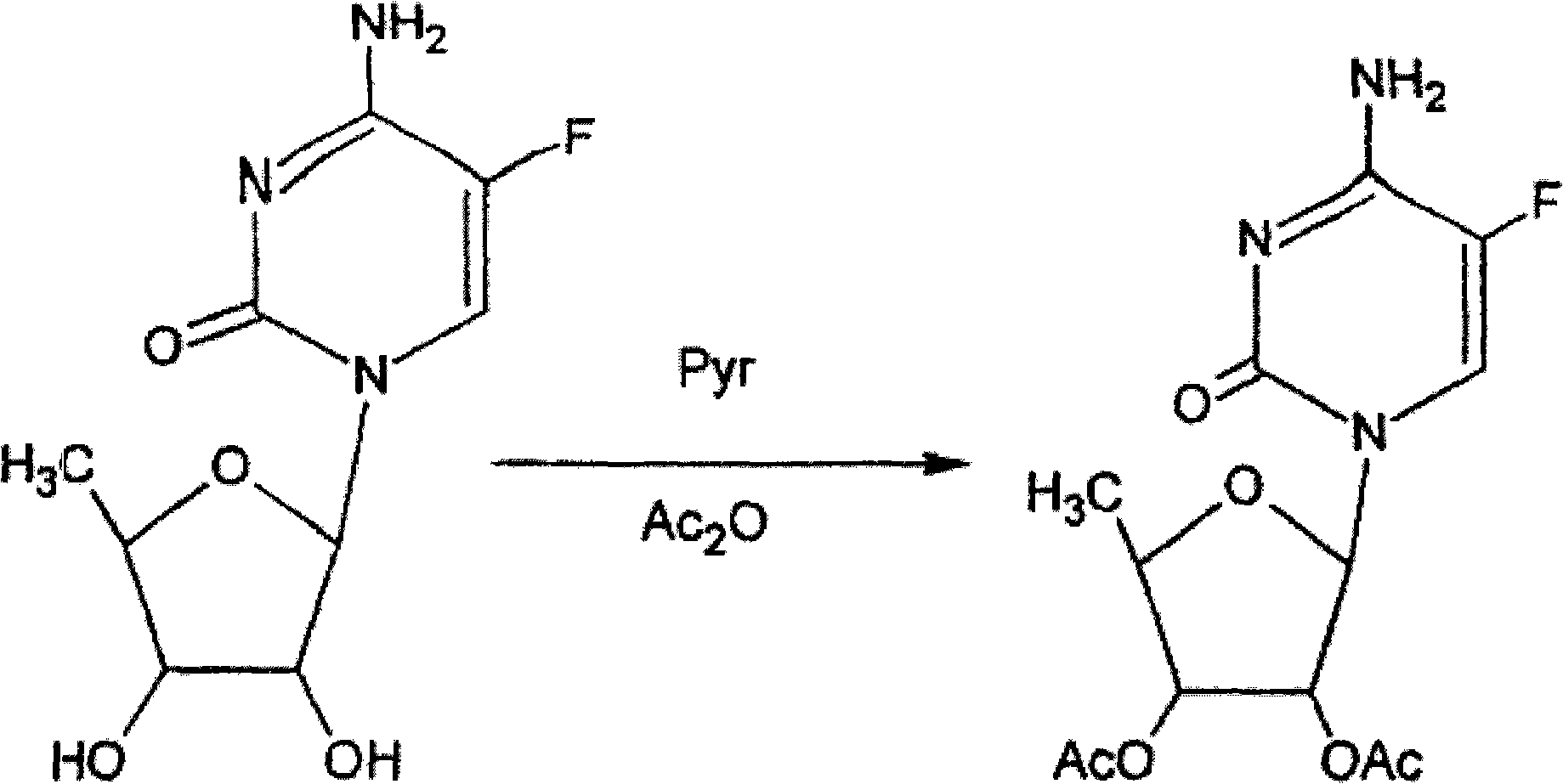
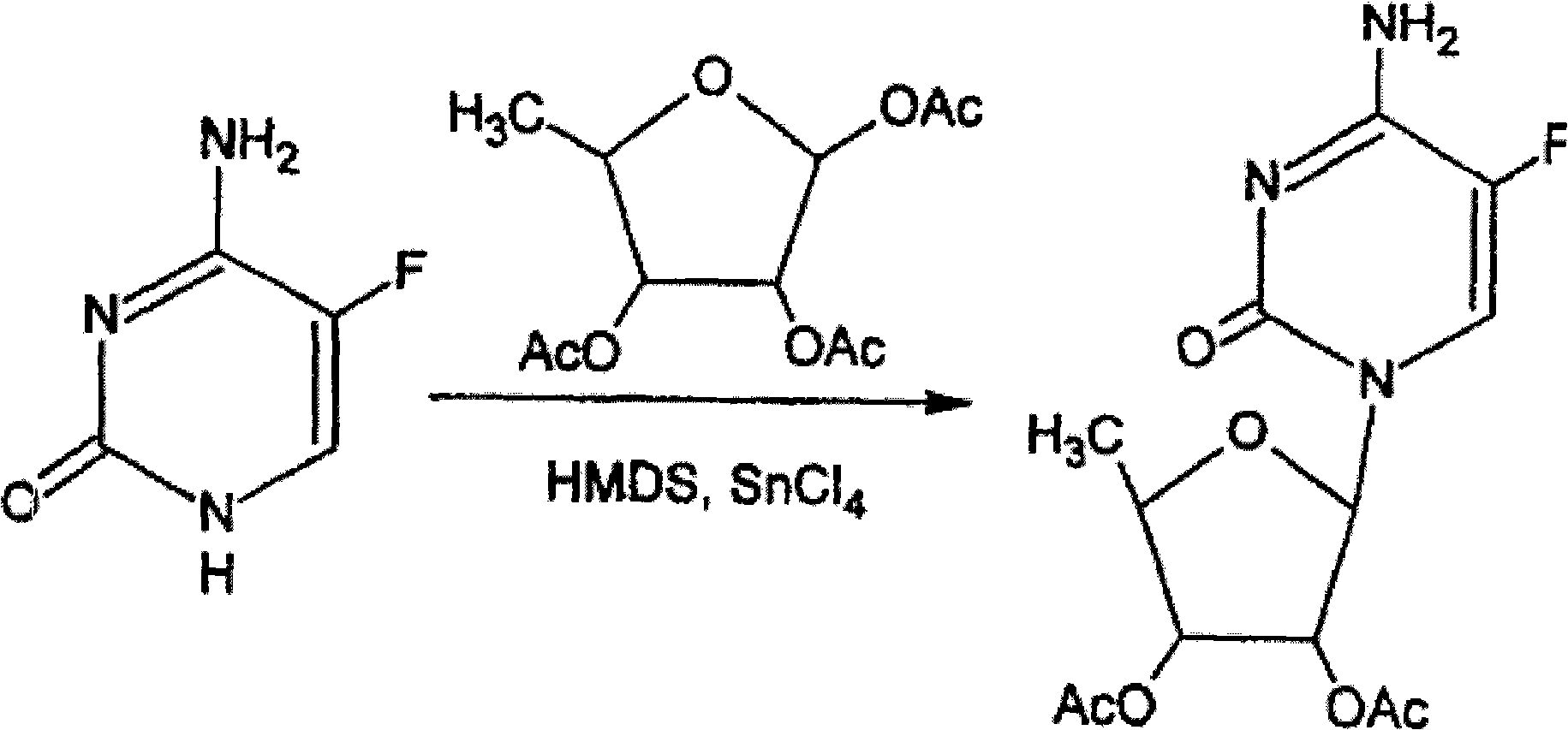
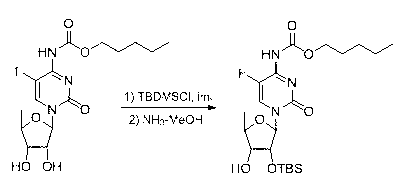
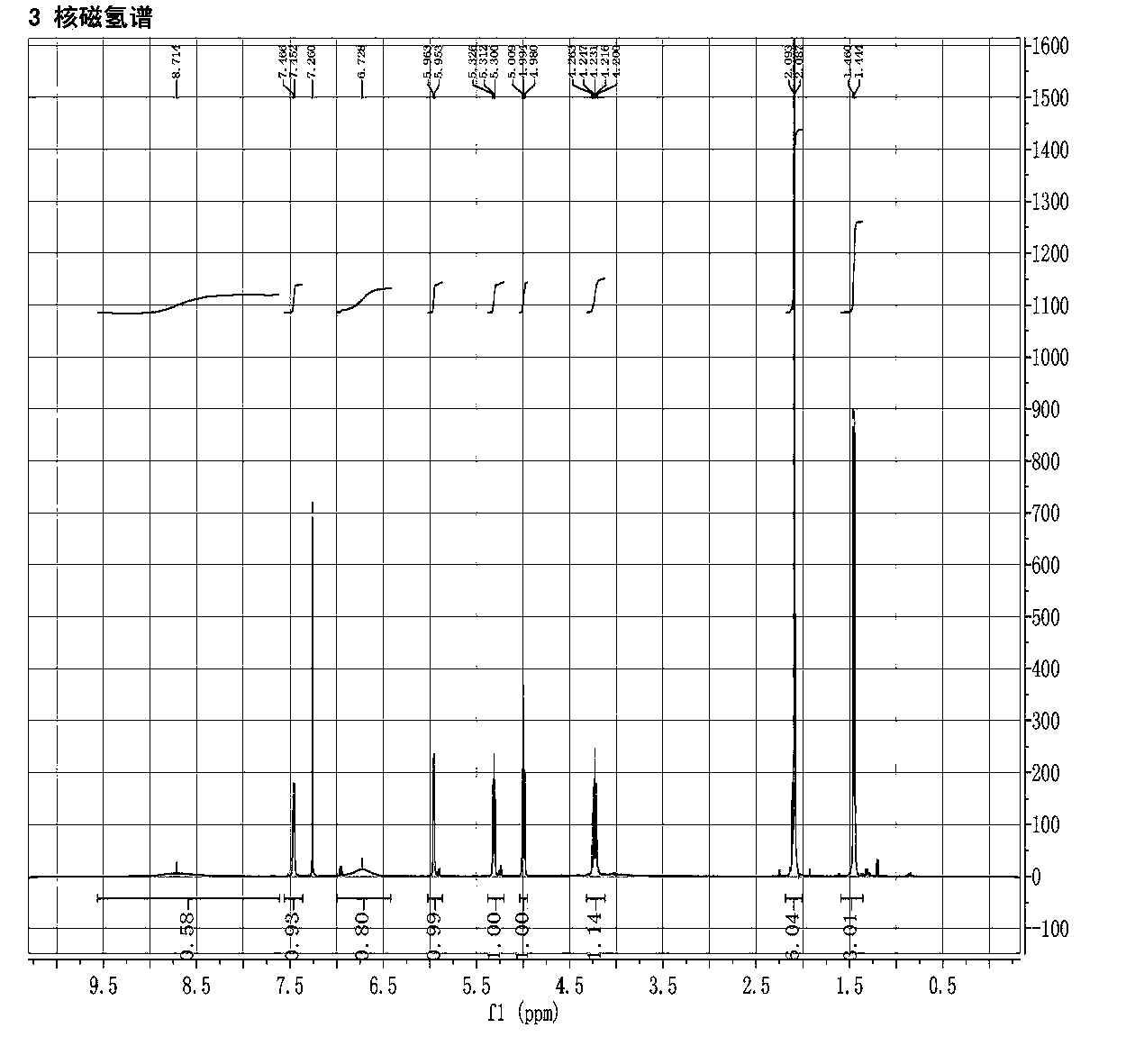
2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method
ActiveCN103524583ARaw materials are easy to getSimple methodSugar derivativesSugar derivatives preparationOrganic solventReaction temperature
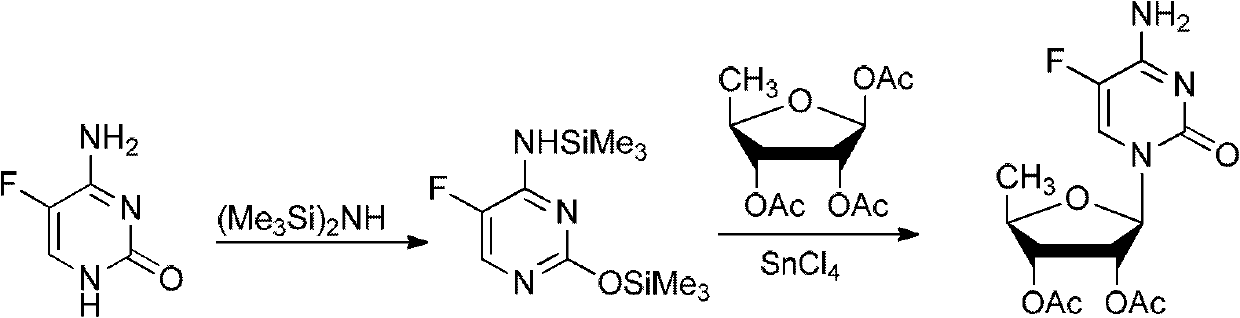
The invention discloses a 2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method. According to the method, 2,3-di-O-acetyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine is adopted as a raw material, and is dissolved by using an organic solvent; organic alkali is added; CO2 is delivered in; and a reaction is allowed under a reaction temperature controlled at 0-50 DEG C and a reaction time controlled at 1-24h. According to the method, an intermediate of a capecitabine production process is used for preparing capecitabine impurity C2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine. The raw materials are easier to obtain, and the method is more simplified.
Owner:SHANDONG BOYUAN PHARM CO LTD
2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound and preparation method thereof
ActiveCN102424697AReduce usageClear structureSugar derivativesSugar derivatives preparationCombinatorial chemistryBiological activation
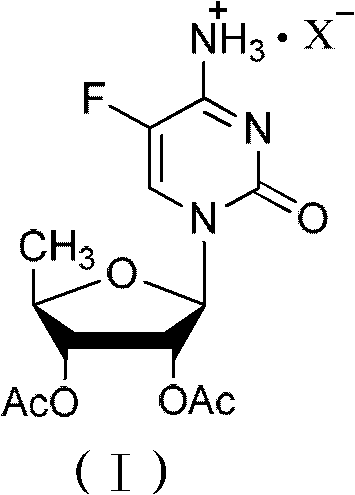
The invention relates to a 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound of formula (I) or a solvate thereof. The compound is prepared by using 5-deoxy-1,2,3-tri-O-acetyl-beta-D-ribofuranose as a raw material, carrying out condensation reaction on 5-deoxy-1,2,3-tri-O-acetyl-beta-D-ribofuranose and protected 5-flucytosine under the activation of iodotrimethylsilane to obtain a reaction product, and carrying out deprotection, acidification and crystallization. According to the invention, a novel intermediate for synthesizing Capecitabine is developed, one pot process is adopted for reacting to obtain the 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound, and high-purity Capecitabine can be further synthesized by using the 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound as a key intermediate. The compound has the characteristics of clear structure, high purity and stable quality. The use of the compound to synthesize Capecitabine can reduce reaction steps, control process cost, and reduce environmental pollution.
Owner:山东安信制药有限公司
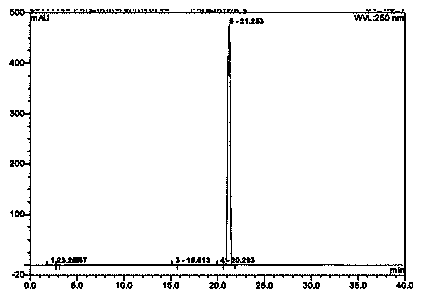
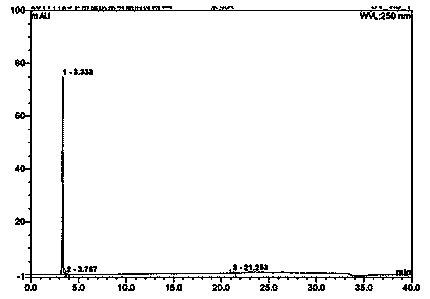
A kind of detection method of capecitabine related substances
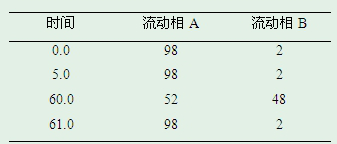
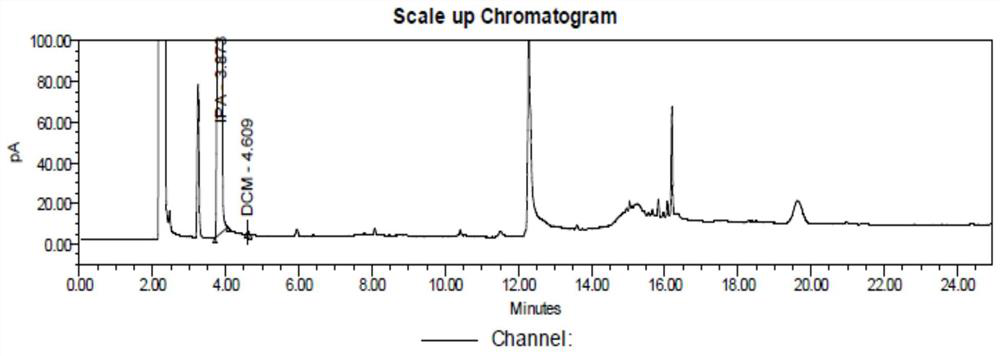
ActiveCN110398555BModerate retention timeEfficient separationComponent separationFluid phaseGradient elution
The invention discloses a detection method for capecitabine related substances: the method adopts high performance liquid chromatography for detection, and includes the following steps: Step 1. Prepare a capecitabine sample solution, the capecitabine The sample solution includes capecitabine test solution and capecitabine control solution; step 2, inject the sample solution obtained in step 1 into a high performance liquid chromatograph, and use mobile phase A and mobile phase B as mobile phases for gradient elution , and record the chromatogram. The detection method adopts a pentafluorophenyl bonded silica gel chromatographic column. The detection method is easy to operate, the retention time of the main peak of capecitabine is suitable, the defects of the prior art are overcome, the impurity D and the impurity E are effectively separated, other known impurities can be effectively detected, and the separation degree is high. It has strong specificity and accurate and reliable detection results, which provides a simple and reasonable detection method for the quality control and impurity research of capecitabine.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Preparation method of capecitabine intermediate suitable for industrial production
PendingCN113321693AReduce processing costsHigh yieldSugar derivativesSugar derivatives preparationSodium bicarbonateDistillation
The invention discloses a preparation method of a capecitabine intermediate suitable for industrial production. The preparation method of the capecitabine intermediate comprises the following steps: carrying out feeding reaction, namely adding acetonitrile, 5-flucytosine, hexamethyldisilazane, trifluoromethanesulfonic acid, 1,2,3-triacetoxy-5-deoxy-D-ribose and trifluoromethanesulfonic acid into a reaction kettle, and performing stirring; carrying out concentrating reaction, namely performing reduced pressure distillation on the solution in the reaction kettle until the volume is 4 times of the reference volume, and adding dichloromethane which is 5-6 times of the reference volume and a sodium bicarbonate solution with the concentration of 8.9 mol / L; carrying out liquid separation and extraction to obtain an aqueous phase and an organic phase; carrying out equal-liquid-level distillation, namely distilling the organic phase until the volume of the liquid is 2.5 times of the volume of the starting material, maintaining the liquid level by continuously replenishing isopropanol in the subsequent distillation process, and replenishing isopropanol to the distillation end-point liquid level after dichloromethane in the system is removed; and then, carrying outcrystallization and filtration drying to obtain the capecitabine intermediate. According to the method, conventional distillation replacement is replaced by equal-liquid-level distillation operation on time; and moreover, the preparation route is further optimized. Thus, the method is suitable for industrial production and popularization of capecitabine intermediates.
Owner:SCINOPHARM CHANGSHU PHARMA
Pharmaceutical composition containing capecitabine and used for treating stomach cancer
The invention relates to an antitumor pharmaceutical composition, particularly relates to a pharmaceutical composition used for treating stomach cancer, and specifically relates to an antitumor pharmaceutical composition containing capecitabine, oleuropein and glycyrrhizin. The molar ratio of the capecitabine to the oleuropein to the glycyrrhizin is sequentially as follows: (8-25): (5-10): (3-8). The three compounds, namely capecitabine, oleuropein and glycyrrhizin in the pharmaceutical composition provided by the invention can mutually generate an obvious synergistic effect, to achieve a better curative effect, so as to reduce the treatment dosage of the capecitabine and reduce the side effects of the capecitabine.
Owner:宋婷婷
Preparation method of capecitabine molecularly imprinted sustained and controlled release material doped with poss and mcm-41-mps
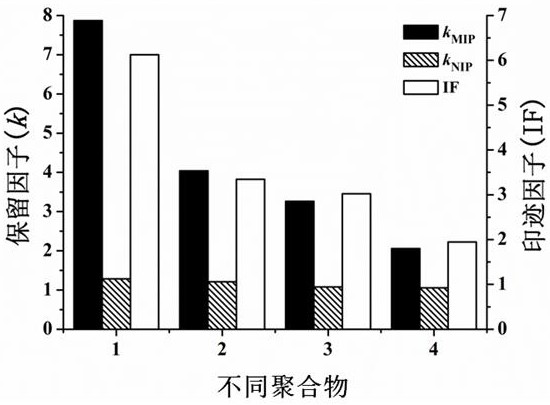
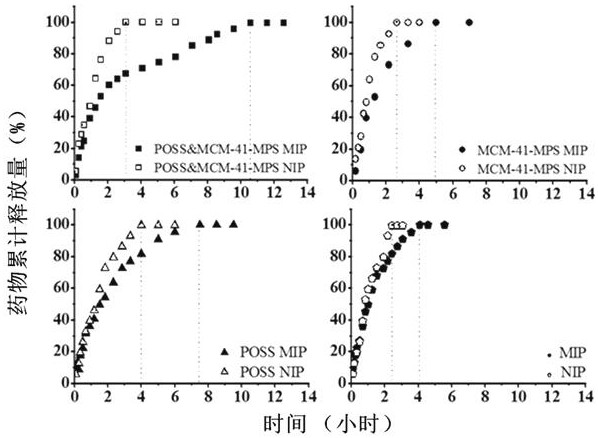
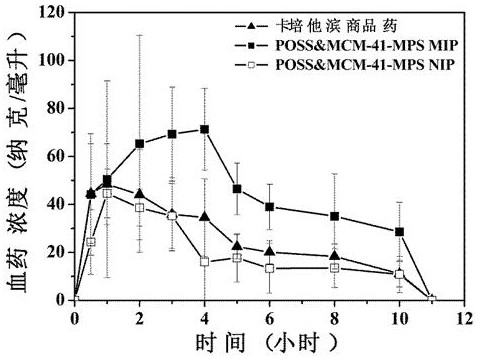
ActiveCN108939082BIncrease the areaImprove adsorption capacityOrganic active ingredientsInorganic non-active ingredientsMolecular sieveDrug release
The invention relates to a preparation method for an integrated POSS and MCM-41-MPS doped capecitabine molecularly-imprinted sustained-release or controlled-release material, specifically to the preparation method and performance evaluation of a capecitabine molecularly-imprinted sustained-release or controlled-release material doped by POSS and the mesoporous molecular sieve MCM-41. Results showthat due to the existence of both POSS and MCM-41-MPS, the selective recognition ability and adsorption performance of a molecularly-imprinted polymer can be significantly improved, better imprintingeffect is generated, and certain influence is performed on the specific surface area and pore size distribution of the polymer. Hybrid MIP is used as a carrier material of capecitabine, and in-vitro and in-vivo drug release experiment results prove that an optimal formula is significantly prolonged in drug release time and significantly improved in relative bioavailability (wherein F is 173.4%), so great research significance and practical value are achieved when the hybrid MIP is applied to the sustained-release or controlled-release material of the capecitabine.
Owner:TIANJIN MEDICAL UNIV
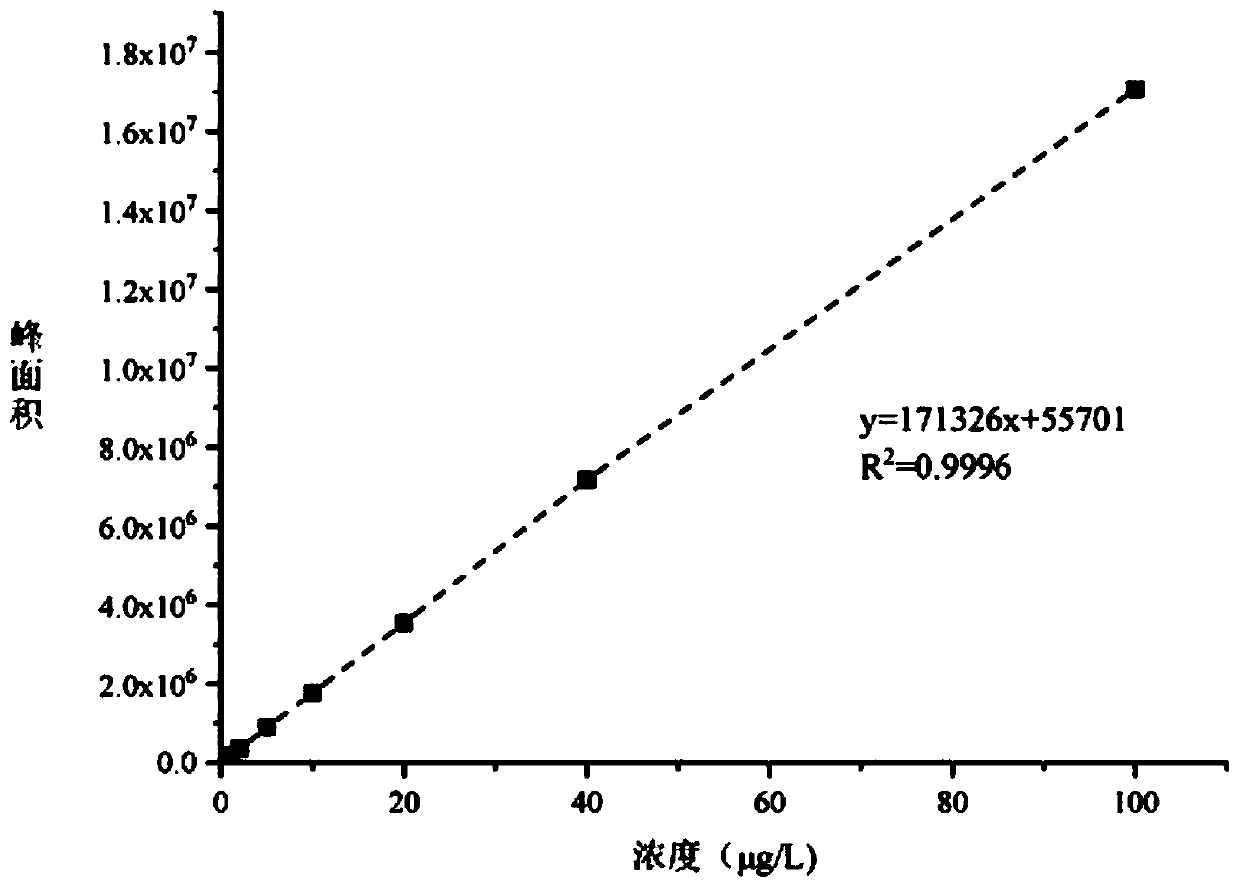
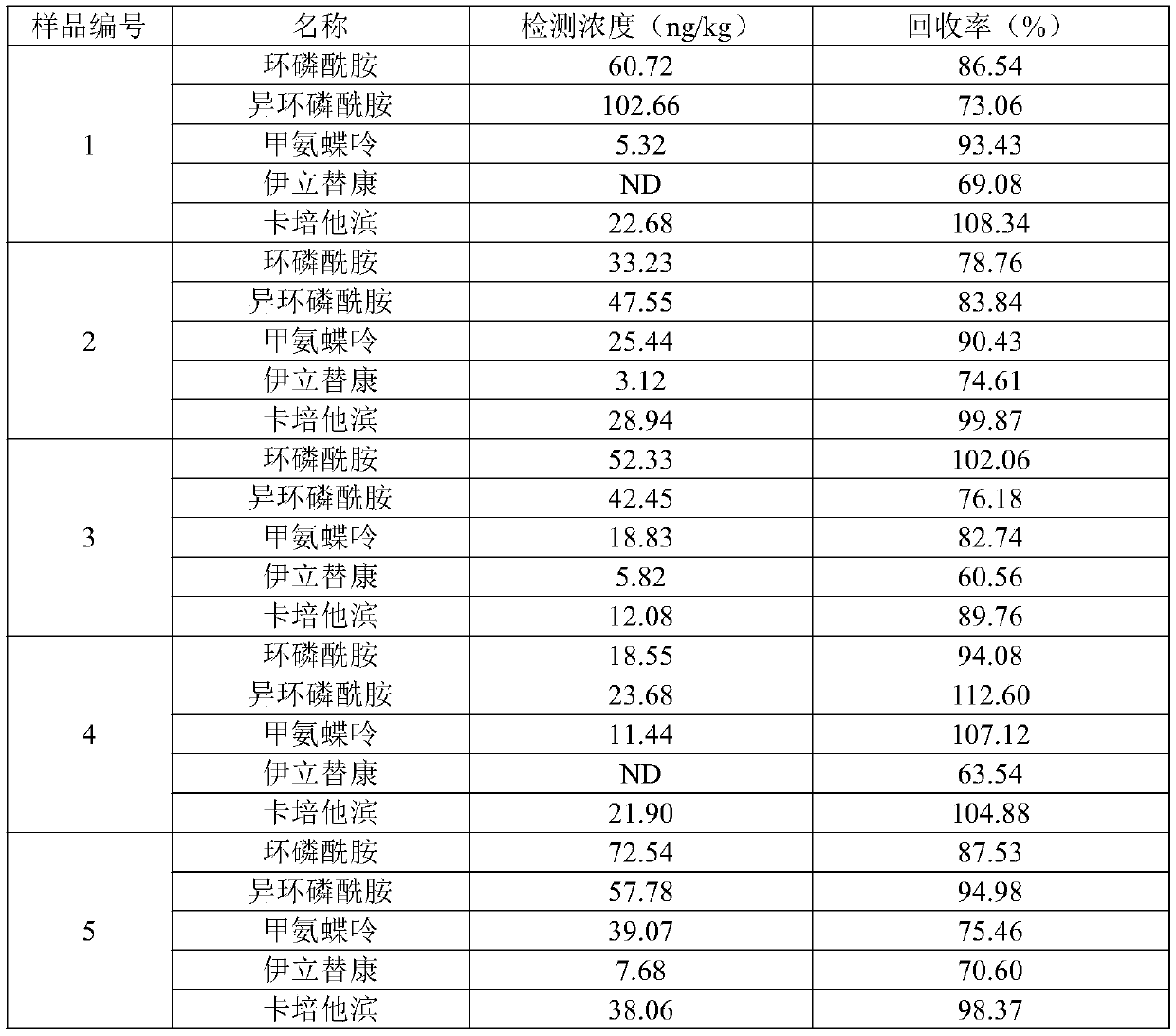
Quick detection and analysis method for anti-cancer drugs in sludge of sewage treatment plant
ActiveCN111551659AImprove stabilityGood repeatabilityComponent separationLiquid chromatography mass spectroscopySludge
The invention discloses a quick detection and analysis method for anti-cancer drugs in sludge of a sewage treatment plant, which can efficiently and quickly detect five anti-cancer drugs in the sludgeat the same time by combining a refrigerated centrifugation-solid phase extraction column with a high performance liquid chromatography-mass spectrometry method, including cyclophosphamide, isocyclophosphamide, methotrexate, irinotecan and capecitabine. The method comprises the following steps: freeze-drying sludge, weighing, adding an internal standard substance, dissolving, centrifuging, blowing nitrogen and redissolving; then extracting and purifying five anti-cancer drugs in the sample by using a solid-phase extraction small column; and detecting the content of the target object in the sample through a high performance liquid chromatography-mass spectrometer. The method is simple in solid sample treatment step, convenient to operate, good in stability, capable of quickly obtaining thesolid sample and suitable for the sample detected by the liquid chromatograph-mass spectrometer; the method is low in sample pretreatment cost, high in detection speed, high in automation degree, sensitive in response, high in recovery rate and convenient for industrial application.
Owner:SHANGHAI UNIV
Preparation method of 5-deoxy-d-ribofuranoside compound
The invention relates to a preparation method of substances 3'-O-(5''-deoxy-beta-D-ribose) capecitabine and 3'-O-(5''-deoxy-alpha-D-ribose) capecitabine relative to capecitabine medicines used for antineoplastic effect, which solves the problem that the impurity generated during a capecitabine production process can be separated from a reaction solution. The method is characterized in that a 5-deoxy-D-ribose donor is prepared, and is subjected to a glycosylation reaction with capecitabine protected by silane, and a protective group is removed to prepare the target product. A double glycosylation related substance can be generated during the production process of capecitabine prepared by a chemical method, and the method provides a qualified reference substance for controlling the quality of capecitabine.
Owner:SHENYANG PHARMA UNIVERSITY
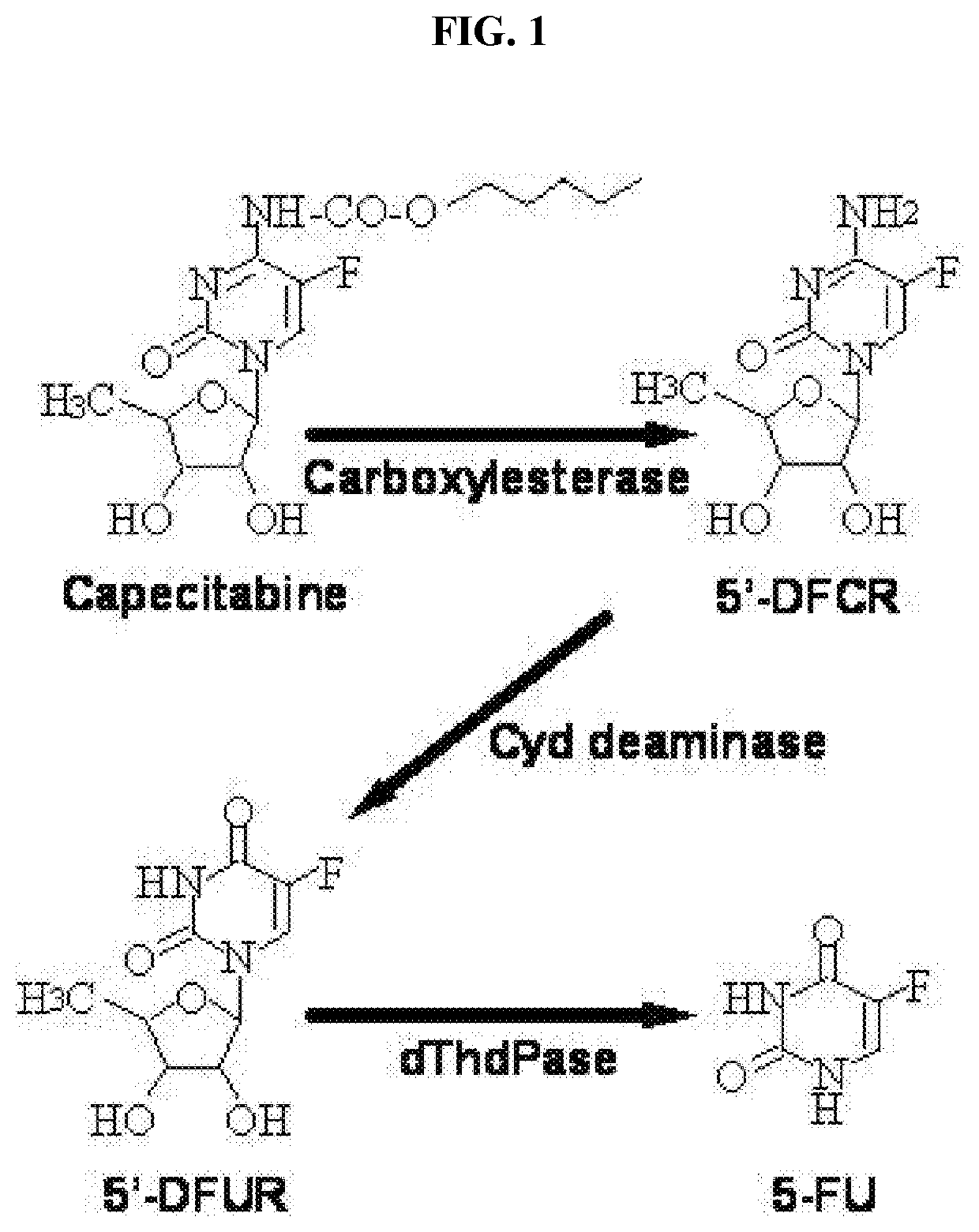
Topical capecitabine for the treatment of hyperproliferative skin conditions
ActiveUS20210052620A1Reduce adverse effectsOrganic active ingredientsOintment deliveryPharmaceutical drugDermatomal
The present invention relates to a novel and unexpected method of using topical Capecitabine composition to obtain therapeutically effective amounts of fluorouracil (FU) within the skin of a subject afflicted with hyperproliferative or inflammatory skin condition. The method comprising topically administering a pharmaceutical composition comprising Capecitabine or a hydrate or solvate thereof to the affected area of the skin of the subject, to form therapeutically effective amounts of FU within the skin.
Owner:TARO PHARMA INDS
Uracil dermatological formulations
PendingCN114423431ACapable of mass productionOrganic active ingredientsOrganic chemistryTG - TriglyceridePyrrolidinones
The present invention provides a topical pharmaceutical formulation comprising uracil and a penetration enhancer and a method of administering the same. Also provided is a method of treating or preventing a skin disease associated with the administration of 5-fluorouracil or a precursor or prodrug thereof, such as capecitabine. The penetration enhancer is selected from the group consisting of dimethyl isosorbide, isopropyl myristate, isopropyl palmitate, octyldodecanol, oleic acid, oleyl alcohol, polyoxyglyceride, pyrrolidone, thymol, trioctyl extract, triolein, myristic acid, medium chain triglyceride, linoleic acid, lauric acid, sugar furfuryl alcohol, glyceryl monooleate, ethyl oleate and dimethyl sulfoxide; and dibutyl sebacate and mixtures thereof.
Owner:纳诺麦缇科斯有限责任公司(经营别称为PHD生物科学
Preparation of capecitabine intermediate
PendingCN113321689AHigh yieldHigh puritySugar derivativesSugar derivatives preparationKetone solventsOrganic base
The invention belongs to the field of drug synthesis, and provides a preparation method of a capecitabine intermediate. Specifically, a compound shown in a formula I reacts with n-amyl haloformate in the presence of a ketone solvent and organic base to obtain a compound shown in a formula II. A large amount of solvent extraction is omitted in post-treatment, the operation is more environmentally friendly, the steps are simple, and the preparation method is very suitable for industrial mass production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
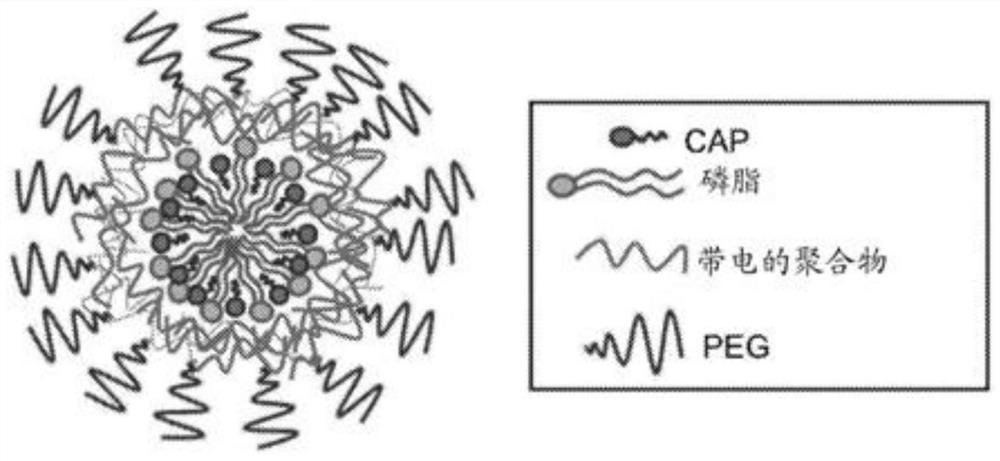
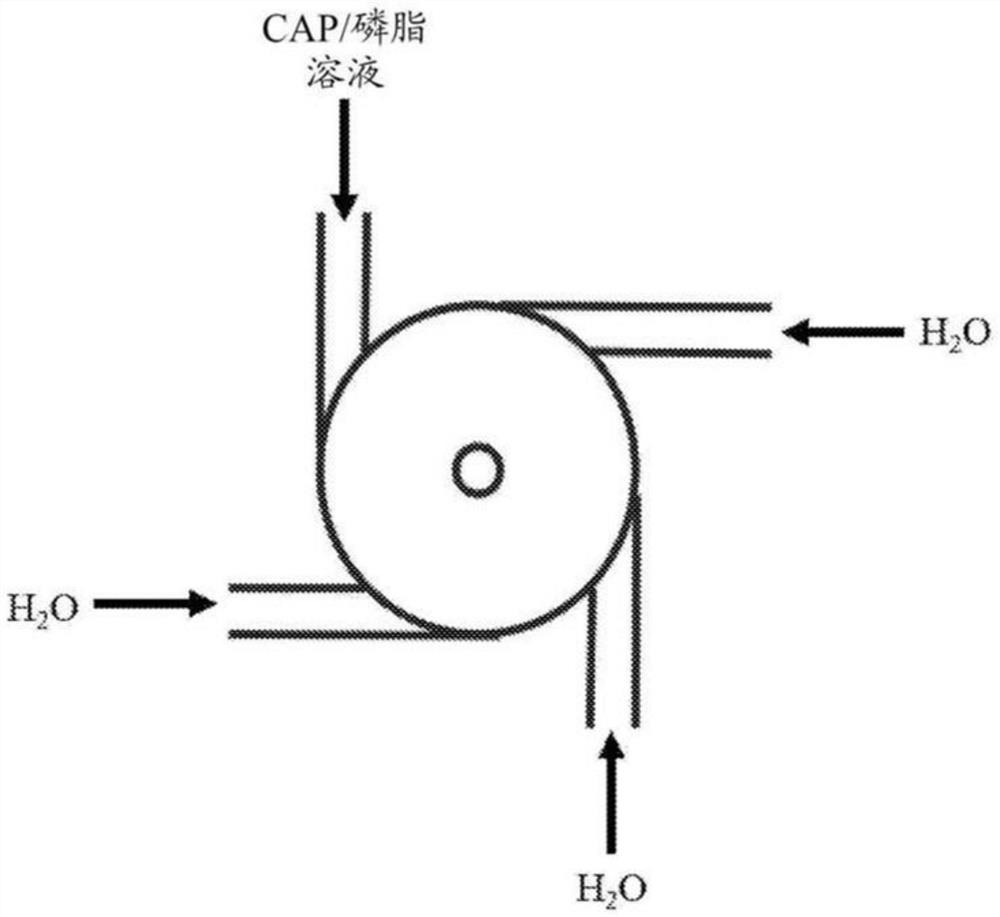
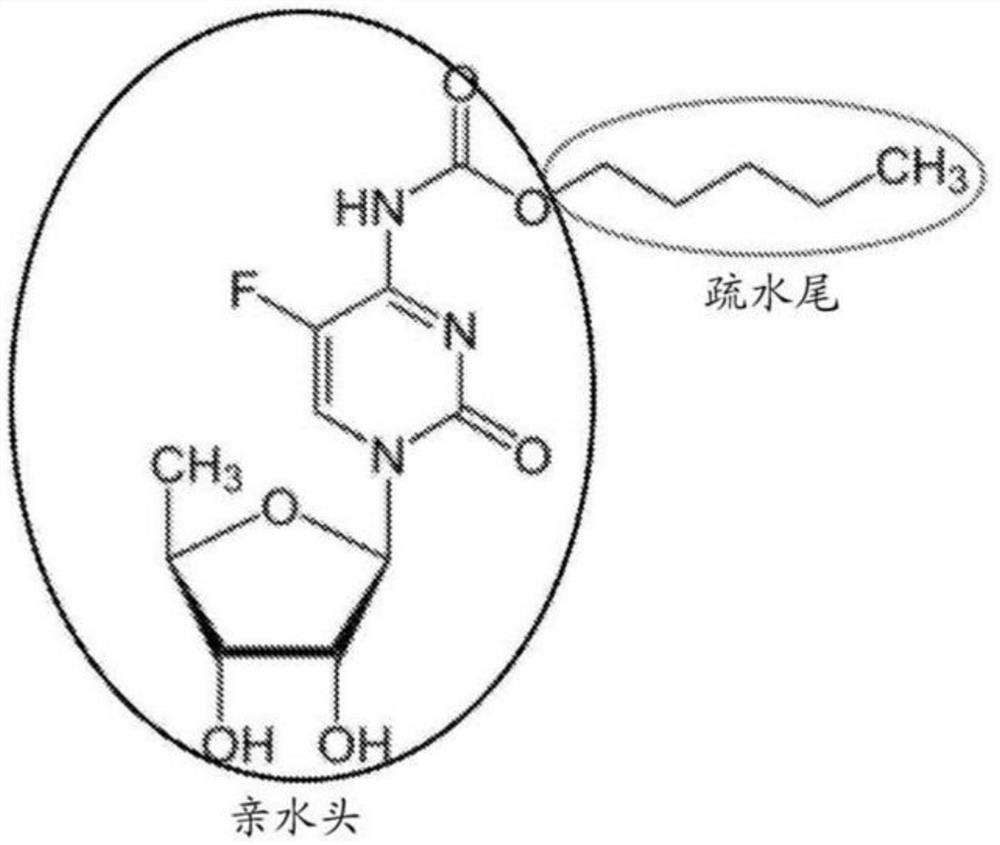
Capecitabine polymer-lipid hybrid nanoparticle capable of utilizing micro-mixing and amphiphilic properties of capecitabine
The invention includes a composition and a method for preparing a nanoparticle composition. The nanoparticle composition comprises a phospholipid core comprising one or more lipids and one or more active agents, and at least one layer of one or more polymers on the surface of the phospholipid core. More specifically, the invention relates to the purpose of the capecitabine (N4-pentyloxycarbonyl-5-deoxy-5-fluoro-cytidine, CAP) in the category of lipid polymer nanoparticle preparations for optimizing the pharmaceutical properties of the capecitabine for treating cancer.
Owner:JINGJIE PTM BIOLAB HANGZHOU CO LTD
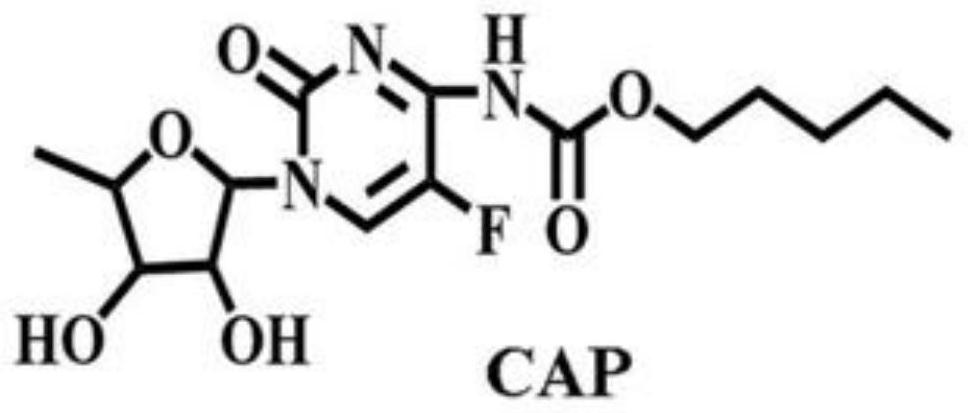
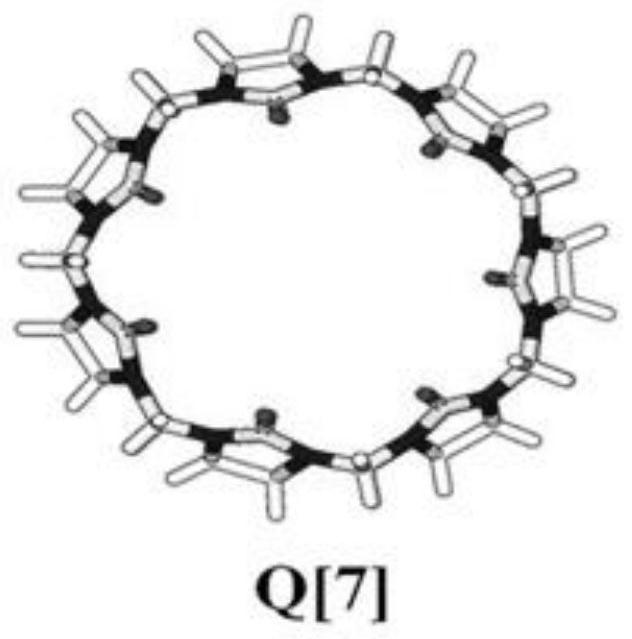
A kind of preparation method of seven-membered cucurbit ring and capecitabine supramolecular inclusion compound
ActiveCN108079313BEffective inclusionSimple and fast operationOrganic active ingredientsPharmaceutical non-active ingredientsPolymer sciencePharmaceutical drug
The invention discloses a preparation method of a seven-membered cucurbit ring and capecitabine supramolecular inclusion compound. The seven-membered melon ring and capecitabine are added to distilled water and mixed to form a mixed solution, and then heated to 45-55°C until the reaction is complete and then cooled to obtain the seven-membered melon ring-capecitabine supramolecular drug inclusion complex things. The invention can prepare the supramolecular clathrate between the seven-membered cucurbit ring and capecitabine, improves the controlled and sustained-release effect of the capecitabine drug molecule in the human body, and has simple preparation method and low cost.
Owner:GUIZHOU UNIV
Nanoscale capecitabine and preparation method thereof
ActiveCN102961342BEasy to prepareLow costOrganic active ingredientsInorganic non-active ingredientsMedicinePhysical chemistry
A nanoscale capecitabine particle and a preparation method therefor. The particle uses silica aerogel as a carrier. Capecitabine is adsorbed in pores of the silica aerogel, forming the capecitabine particle having a diameter of 100 nm or less. The silica aerogel has a porosity of 95-99%, an aperture of 10-50 nm, a specific surface area of 200-1000 m2 / g, and a density of 3-300 kg / m3, and the diameters of gel particles forming a network are 1-50 nm.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
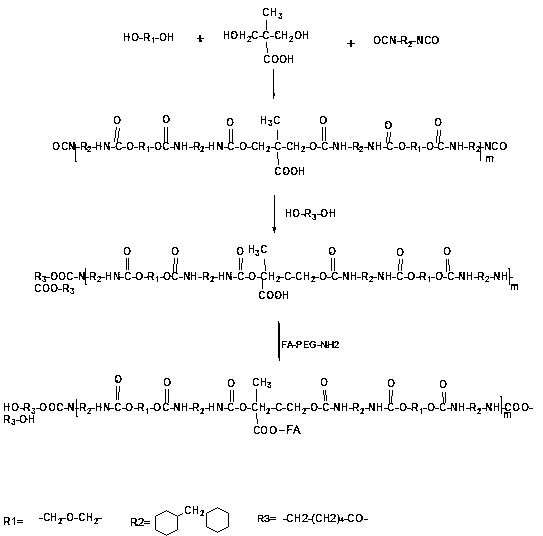


![2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method 2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method](https://images-eureka.patsnap.com/patent_img/c06c38de-05b9-4511-a7a8-d855e4fab1c4/2013105233412100002DEST_PATH_IMAGE001.PNG)
![2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method 2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method](https://images-eureka.patsnap.com/patent_img/c06c38de-05b9-4511-a7a8-d855e4fab1c4/2013105233412100002DEST_PATH_IMAGE004.PNG)
![2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method 2',3'-O-carbonyl-5'-deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine synthesis method](https://images-eureka.patsnap.com/patent_img/c06c38de-05b9-4511-a7a8-d855e4fab1c4/38479DEST_PATH_IMAGE002.PNG)